Screening of antidote sensitivity using an acetylcholinesterase biosensor based on a graphene–Au nanocomposite
Abstract
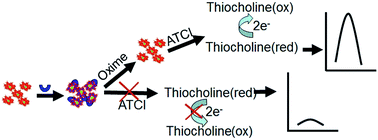
In this paper, we developed a new technique for in vitro screening of the therapeutic effects of three oxime compounds: obidoxime, 2-pralidoxime methiodide (2-PAM-I) and pyridine-2-aldoxime methochloride (2-PAM-Cl). Acetylcholinesterase (AChE) is first immobilized in a thin nanocomposite film composed of graphene and gold nanoparticles on a glassy carbon electrode by a self-assembly approach. The immobilized AChE in the nanocomposite is first exposed to an organophosphate agent, paraoxon-ethyl, and the enzyme activity is inhibited. The therapeutic effect is based on the reactivation capability of the oximes. AChE activity is measured by an electrochemical method. Our study indicated that oximes' reactivation capacity is correlated to their molecular structures. Obidoxime, having dual oxime groups, showed higher reactivation capacity than 2-PAM compounds with a single oxime group. Due to the similar molecular structures of 2-PAM-I and 2-PAM-Cl, a slight difference between their reactivation capacities was found, and this difference is attributed to the stronger electron withdrawing ability of iodine ions than that of chloride ions. The proposed electrochemical method thus provides a new simple tool for new drug screening.


 Please wait while we load your content...
Please wait while we load your content...