Mild fabrication of silica-silver nanocomposites as active platforms for environmental remediation†
A. Mignani*a,
S. Fazzinib,
B. Ballarin*b,
E. Boaninic,
M. C. Cassanib,
C. Maccatod,
D. Barrecae and
D. Nannib
aCenter for Industrial Research – Advanced Applications in Mechanical Engineering and Materials Technology (CIRI-MAM), University of Bologna, Viale del Risorgimento, 2 I-40136 Bologna, Italy. E-mail: a.mignani@unibo.it; Fax: +39 0512093690; Tel: +39 0512093704
bDepartment of Industrial Chemistry “Toso Montanari”, University of Bologna and INSTM, Viale del Risorgiento, 4 I-40136 Bologna, Italy. E-mail: barbara.ballarin@unibo.it
cDepartment of Chemistry “Giacomo Ciamician”, University of Bologna, Via Selmi, 2 I-40126 Bologna, Italy
dDepartment of Chemical Sciences, University of Padova and INSTM, Via Marzolo 1, I-35131 Padova, Italy
eCNR-IENI and INSTM, c/o Department of Chemical Sciences, University of Padova, Via Marzolo 1, I-35131 Padova, Italy
First published on 6th January 2015
Abstract
Herein we report a new, simple, low cost and one step way to obtain silica-supported silver nanoparticles (AgNPs) on commercial polyethyleneimine-functionalized silica beads (SiO2-PEI) under mild experimental conditions. The novel AgNPs/(SiO2-PEI) material has been thoroughly analyzed using FE-SEM, BET, XRD, XPS and XE-AES analysis. The reduction of Methylene Blue (MB) to Leuco Methylene Blue (LMB) in the presence of NaBH4 was chosen for testing the catalytic properties of AgNPs/(SiO2-PEI) towards dyes decoloration. Moreover, the prepared supported nanocatalyst was also found to exhibit excellent catalytic activity towards decoloration of some azo dyes such as E110 and E122.
1. Introduction
Dyes and pigments that contain heterocyclic aromatic compounds are normally present in the waste waters of textiles, paper, cosmetics and leather industries. They are very dangerous to the environment and human health1 and, for example, prolonged exposure to them causes irritation of the respiratory system and the gastro-intestinal track. Moreover, most of these dyes (in particular azo dyes) are highly recalcitrant to conventional waste water biological treatment2 and pretreatment processes are required to decolorize them efficiently. Many methods are available for this purpose, such as oxidation (with ozone, H2O2 or chlorine dioxide), Fenton, photo Fenton, or electrochemical advanced Fenton oxidation, but they are expensive, require harsh working pH ranges or can generate a large volume of ferrous slurry.3 Photocatalytic materials such as TiO2 have been even largely employed to achieve the thorough degradation of azo dyes, but normally they are active only in the UV range because of their wide band gap.4 Thus the development of simple and low cost materials/methods for efficient dye degradation has gained enormous significance. In this context extensive research has been carried out to explore the use of noble metal nanoparticles (NPs i.e. Au or Ag) for catalytic reduction of dyes, due to their enhanced catalytic activity.5 Additionally, due to the interest and progress in heterogeneous catalysis, significant attention has been focused on the possibility of stabilizing the NPs onto solid supports such as silica, indium tin oxide, alumina, graphene oxide, etc.5 with the purpose of removing and recycling them, after the decoloration treatment, in a simple way. Recently, detailed investigations into the syntheses of silver nanoparticles (AgNPs), with tunable size and shape, have drawn much attention.6–8On the basis of our previous results,9 in this paper we propose an innovative, simple and low cost procedure for preparing silica-supported AgNPs by using AgNO3 and commercial polyethyleneimine functionalized silica beads (SiO2-PEI), under mild conditions.
Polyethyleneimine (PEI) is an interesting material for reducing and stabilizing metal NPs; it is a cationic polymer with a high charge density. PEI fragments containing amino groups can easily chelate with metal ions, and they can also act as both reducing agents and stabilizers in preparation of metal NPs. Indeed, rapid formation of silver nanoparticles occurred within few minutes by heating the solution, indicating that the heat treatment promotes the direct redox reaction between PEI and AgNO3, without the additional step of introducing other reducing and/or protective agents.10 Silica beads provide high surface area, give excellent mechanical strength and offer thermal stability. We have investigated the effect of the silver nitrate precursors concentrations and of the reaction times with the aim to optimize the synthesis of AgNPs/(SiO2-PEI) catalyst. All the samples have been characterized by X-ray Fluorescence (XRF), BET surface area measurements, Field Emission-Scanning Electron Microscopy (FE-SEM), X-ray Diffraction (XRD), X-ray Photoelectron and X-ray Excited Auger Electron Spectroscopy (XPS and XE-AES).
The reduction of Methylene Blue (MB) to Leuco Methylene Blue (LMB) in presence of a reducing agent such as NaBH4 (ref. 11) was chosen for testing the catalytic properties of AgNPs/(SiO2-PEI) towards dyes decoloration. Finally, AgNPs/(SiO2-PEI) have been successfully used as catalysts to decolorize azo-dyes, such as Sunset Yellow and Azorubine.
2. Experimental section
2.1. Materials
AgNO3 and NaBH4 were purchased from Sigma-Aldrich and used without further purification; ultrapure water purified with the Milli-Q plus system (Millipore Co., resistivity over 18 MΩ cm) was used in all cases. Commercial silica functionalized with polyethylenimine (SiO2-PEI) (Mw 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000−50
000−50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Sigma-Aldrich as 20–60 mesh orange beads. Methylene Blue trihydrate, Sunset Yellow (E110) and Azorubine (E122) were purchased from Sigma Aldrich.
000) was purchased from Sigma-Aldrich as 20–60 mesh orange beads. Methylene Blue trihydrate, Sunset Yellow (E110) and Azorubine (E122) were purchased from Sigma Aldrich.
2.2. Preparation of AgNPs/SiO2-PEI catalyst
The commercial SiO2-PEI beads were preliminary treated with 1.3 M KNO3 for 6 h under stirring to eliminate chlorides (present as impurity) via anionic exchange; their presence may in fact cause problems during the AgNPs/SiO2-PEI preparation, giving rise to silver chloride precipitation as shown by XRD pattern (see ESI, Fig. S1†). Hence 0.500 g of SiO2-PEI were added to an aqueous solution (50 mL) of 41.2 mM AgNO3 (PEI/Ag molar ratio equal to 0.025), previously heated at 100 °C. The resulting suspension was kept at 100 °C under stirring at 500 rpm speed for different times (1, 2 or 3 h). In a few minutes, the orange beads turned brown due to the adsorption of silver on SiO2-PEI and subsequently no changes are observed even after the silver reduction. The AgNPs/(SiO2-PEI) beads were collected by filtration on a buchner funnel and washed several times with water. This last procedure was carried out until a test with sodium chloride did not show any AgCl precipitate in the washing water. Then AgNPs/(SiO2-PEI) beads were dried under vacuum for 24 h at room temperature.2.3. Instruments
FE-SEM measurements were carried out with a Zeiss SUPRA 40VP instrument at a primary beam acceleration voltage of 10 kV; micrographs were collected with an InLens detector. Prior to FE-SEM measurements, samples were coated with 10 nm thick carbon films by a Sputter-Coater (EDWARDS) to suppress charging phenomena leading to image distortions. ImageJ® picture analyzer software was used to estimate the average nanoparticles dimensions.12 XRD analysis was carried out by means of a PANalytical X'Pert PRO powder diffractometer equipped with a fast X'Celerator detector. Ni-filtered CuKα radiation was used (λ = 0.154 nm, 40 mA, 40 kV). For phase identification the 2θ range was investigated from 15 to 60 2θ degrees with a step size of 0.2° and time per step of 1000 s. The amount of silver on the different samples was determined with a wavelength dispersive XRF instrument (Panalytical Axios Advanced) by comparison with calibration lines, obtained by incipient impregnation adding aqueous solutions of AgNO3 to 500 mg of SiO2-PEI matrix. Successively, ca. 145 mg of either standard samples were mixed with about 100 mg of CEREOX Licowax C binding wax micropowder. The resulting solid was ground into a fine powder and pelletized into a 13 mm diameter disk. Each analysis was repeated five times. The specific surface areas of AgNPs/(SiO2-PEI) samples and of the pristine SiO2-PEI before and after the heating process at 100 °C and the pre-treatment in 1.3 M KNO3 (described in paragraph 2.2) were measured by N2 adsorption–desorption, with BET isotherm equation, using a Sorpty 1750 (Carlo Erba) analyzer. The sample was degassed under vacuum at 90 °C before analysis. XPS and XE-AES analyses were run on a Perkin Elmer Φ 5600ci spectrometer at a working pressure lower than 10−8 mbar, using a non-monochromatized AlKα excitation source (hν = 1486.6 eV). The spectrometer was calibrated by assigning to the Au4f7/2 line the Binding Energy (BE) of 84.0 eV with respect to the Fermi level. Charging correction was performed by assigning to the C1s line of adventitious carbon a value of 284.8 eV.13,14 The estimated standard deviation for BEs was ±0.2 eV. After a Shirley-type background subtraction,15 raw spectra were fitted by means of a non-linear least-squares deconvolution program, using Gaussian–Lorentzian peak shapes. Atomic percentages were evaluated using sensitivity factors provided by Φ V5.4A software. The samples were introduced directly into the analysis chamber by a fast entry lock system.2.4. Catalytic activity (decoloration treatment)
The catalytic activity of AgNPs/(SiO2-PEI) towards the reduction of different dyes in the presence of an excess of NaBH4 at 25 °C, was followed by UV-vis measurements with a UV-vis single beam Hewlett-Packard 8453 diode array spectrophotometer equipped with a 10 mm quartz cuvette.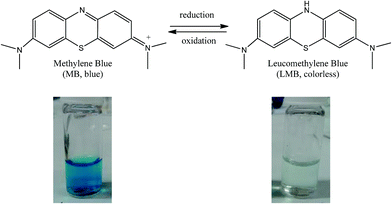 | ||
| Scheme 1 Reduction reaction of MB to LMB and photographs of the vials at time 0 s (left) and after 300 s (right) in presence of AgNPs/(SiO2-PEI), MB and NaBH4. | ||
In a typical experiment, 1 mL of MB solution (9.4 × 10−5 M) was purged with N2 gas for about 5 min to remove all dissolved oxygen. Then, the AgNPs/(SiO2-PEI) and 2 mL of freshly prepared NaBH4 were added (the following concentrations were tested: 8 × 10−2 M, 2 × 10−2 M, 4 × 10−3 M). The evolution of the reaction was monitored by UV-vis measurements at 25 °C. No color change was observed when the reaction was performed in the absence of AgNPs/(SiO2-PEI).
3. Results and discussion
3.1. SiO2-PEI and AgNPs/SiO2-PEI preparation and characterization
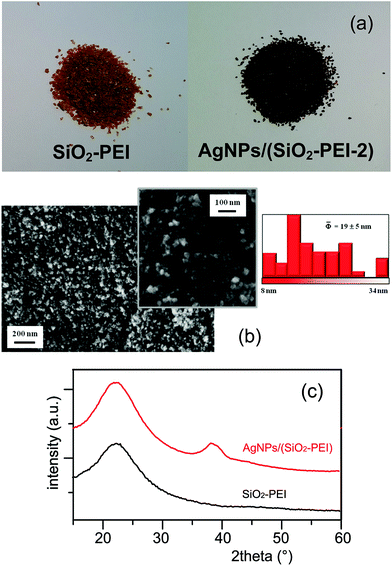
The commercial SiO2-PEI beads have been characterized and the most relevant data obtained are: 12 wt% of organic material and 0.103 mmol g−1 of amino groups present on the silica surface. SiO2-PEI beads was pretreated with 1.3 M KNO3 under stirring for 6 h to allow chlorides removal via anionic exchange. After this pretreatment the specific surface area of commercial SiO2-PEI is 210 m2 g−1 and it resulted stable independently of the heating process (normally at 100 °C in MilliQ water for 2 h).AgNPs/SiO2-PEI was obtained by simple addition of 0.500 g of SiO2-PEI to a stirred aqueous solution (50 mL) of 41.2 mM AgNO3 (PEI/Ag molar ratio equal to 0.025), previously heated at 100 °C, for a reaction time of 2 h under stirring [sample hereafter termed AgNPs/(SiO2-PEI-2)]. PEI acts as a non-toxic reducing agent of silver ions due to the reduction properties of the amino functional groups present in the polymer; it allows for controlling both nucleation and growth process at the same time, playing the role of protective agent for the formed NPs.16 The morphology of AgNPs/(SiO2-PEI-2) powders (Fig. 1(a)) was investigated by FE-SEM after coating the surface with a 10 nm thick carbon film. FE-SEM images, reported in Fig. 1(b), show the presence of raspberry-like aggregates characterized by an homogeneous coverage of the SiO2-PEI surface with highly dispersed silver nanoparticles, hence validating the effectiveness of the adopted preparation process in obtaining a controlled Ag aggregate distribution. The average size of silver-containing aggregates was estimated to be 9 ± 2 nm.
The presence of silver was also confirmed by XRD patterns of AgNPs/(SiO2-PEI-2) powder as reported in Fig. 1(b). The comparison with the amorphous pattern of bare SiO2-PEI reveals the diffraction peak at approximately 38.1° associated with the (1 1 1) plane of the silver crystal (ICDD PDF 01-089-3722). The large broadening of this peak is due to the very low dimensions of coherently scattering domains, which is related to the nanometric dimensions of crystal size.
The total amount of silver present on AgNPs/(SiO2-PEI-2) was estimated to be 1.33 wt% by XRF analysis, as an average of three different prepared samples. Furthermore, a specific surface area of 260 m2 g−1 was obtained by BET measurements.
The XPS survey spectrum of AgNPs/(SiO2-PEI-2) indicates the presence of carbon, oxygen, silver, silicon and nitrogen. No other elements were detected in appreciable amounts (ESI, Fig. S2,† spectrum A). The analysis of the higher resolution surface C1s, O1s, Ag3d, Si2s, N1s and AgMNN regions allowed a more detailed insight into the sample composition (ESI, Fig. S3,† signals A, B and C) and enabled to extract atomic percentage values reported in Table S1 (see ESI†). Furthermore, it also allowed to establish that silver was mainly present in its metallic state, although the minority co-existence of small Ag(I) amounts could be evidenced by calculation of the silver Auger α parameters. More spectroscopic details on XPS and XE-AES investigation are presented and discussed in ESI,† Paragraph 2.
3.2. AgNPs/SiO2-PEI preparation: reaction time effect
Two different reaction times were even investigated (i.e. 1 and 3 h in respect of the 2 h previously discussed; samples AgNPs/(SiO2-PEI-1) and AgNPs/(SiO2-PEI-3), respectively). No significant morphologic differences were observed by FE-SEM images (not reported). In Table 1 the total amount of silver and the specific surface area are reported for the three different samples. Even in this case the differences are quite insignificant and suggest that nucleation and growth of AgNPs occurs during the first hour of reaction.| Sample name | Experimental conditions | Ag (wt%) | Specific surface area (m2 g−1) |
|---|---|---|---|
| AgNPs/(SiO2-PEI-1) | PEI/Ag 0.025, 100 °C, 1 h | 1.26 | 265 |
| AgNPs/(SiO2-PEI-2) | PEI/Ag 0.025, 100 °C, 2 h | 1.33 | 260 |
| AgNPs/(SiO2-PEI-3) | PEI/Ag 0.025, 100 °C, 3 h | 1.57 | 271 |
3.3. Catalytic applications
| ln(At/A0) = −kt | (1) |
The MB reduction in pseudo-first-order conditions took place very fast using AgNPs as a catalyst. The UV-vis absorption band at 665 nm for MB decreased gradually in time without showing any changes in shape or position of the peak, indicating that MB is reduced without any other side reactions, as it can be seen in Fig. 3(a). In the presence of SiO2/PEI only, the peak at 665 nm remained almost unaltered even after 2000 s, Fig. 3(b).
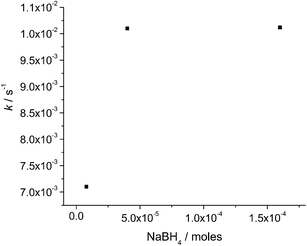
Moreover, with increasing of the catalyst amount (AgNPs/(SiO2-PEI-2)), keeping constant the MB/NaBH4 molar ratio (i.e. 1/1700) it is possible to observe an increase of k. Since the catalytic effect is due to the silver present in AgNPs/(SiO2-PEI-2) catalyst, in Fig. 4 we show the moles of silver, estimated by XRF analysis. A significant increase in kinetic constant values (around 10 times) occurred when the amount of silver changes from 0.75 to 0.99 μmoles (reaction condition: 1/8/1700 and 1/10.5/1700 MB/Ag/NaBH4 molar ratio, respectively); a plateau was achieved for higher quantity of silver.
In Table 2 are summarized the kinetic constants k and the R2 values obtained for all the catalytic tests carried out.
| Entry | MB/Ag/NaBH4 (moles/moles/moles) | Reaction time (s) | ka (10−2 s−1) | R2 |
|---|---|---|---|---|
| a Average ± S.D., n = 3 measures. | ||||
| 1 | 1/4.4/1700 | 500 | 0.80 ± 0.05 | 0.95 |
| 2 | 1/5.4/1700 | 500 | 0.90 ± 0.12 | 0.97 |
| 3 | 1/8/1700 | 150 | 1.03 ± 0.07 | 0.98 |
| 4 | 1/9/1700 | 150 | 2.11 ± 0.15 | 0.98 |
| 5 | 1/10.5/1700 | 30 | 7.67 ± 0.09 | 0.99 |
| 6 | 1/12/1700 | 30 | 7.65 ± 0.11 | 0.99 |
It is worth noting that k values shown in this work are comparable and, in some cases (entries 5 and 6 in Table 2), higher than those usually reported in the literature (see ESI, Table S2†).18–20
By plotting the absorbance at λmax (665 nm, Aλmax) vs. time a sigmoidal curve was obtained. As shown in Fig. 5 an induction period, i.e. the time required to start the catalytic reduction and detect an appreciable change in the absorbance, was observed, and it is especially evident for entry 1 in which the lowest silver amount was employed.
 | ||
| Fig. 5 Plots A at λmax vs. time for AgNPs/(SiO2-PEI-2) with MB/Ag/NaBH4 molar ratio: (a) 1/10.5/1700 (b) 1/9/1700 (c) 1/4.4/1700. | ||
Finally, by comparing the plots Aλmax vs. time obtained for 1/8/425 and 1/8/1700 MB/Ag/NaBH4 molar ratios, an increase of the induction time at about 200 s was also observed when a lower amount of NaBH4 was used (ESI, Fig. S5†). This behavior is in keeping with previously reported data.21–23
 | ||
| Scheme 2 Sunset Yellow and Azorubine. Inset: photographs of vials with SY and AZ (a and c) before and (b and d) after decoloration. | ||
Sunset Yellow UV-vis spectrum shows a characteristic strong visible band with λmax = 485 nm (ref. 24) while Azorubine UV-vis absorption spectrum shows a characteristic band at λmax = 510 nm,25 in ESI, Fig. S6,† (a) and (b). The SY and AZ reduction reactions in presence of AgNPs/(SiO2-PEI-2) and under the pseudo-first-order condition employed for MB experiments (1/4/1700 dye/Ag/NaBH4 molar ratio) took place very rapidly (ca. 1–2 s). No change in color, even after 2000 s, was ever observed using only SiO2-PEI under similar experimental conditions.
In order to follow the catalytic kinetic via UV-vis (ESI, Fig. S7,† a and b) we decreased the amount of reducing agent and we employed for all the measurements a 1/4/21 dye/Ag/NaBH4 molar ratio (1.9 × 10−7 moles dye, 4.0 × 10−6 moles NaBH4, 8.0 × 10−7 moles Ag). Under these conditions, k values of (1.4 ± 0.3) × 10−2 s−1 and (0.28 ± 0.03) × 10−2 s−1 for SY and AZ were obtained, respectively.
3.4. Recycling tests
The catalytic performances of recycled samples were tested sequentially three times for MB and SY dyes. Before reusing it, the recycled catalyst was washed three times with copious water and a 0.3 M solution of sodium carbonate in order to favor removal of borates and residual reaction products. As it can be observed in Table 3, a decrease of the kinetic constant values in each of the subsequent cycles occurred. In order to investigate the cause of this behavior further investigations were carried out on the used catalysts. FE-SEM analysis effected after the first catalytic cycle showed an appreciable increase of the mean AgNPs size: i.e. from 9 ± 2 nm to 19 ± 5 nm and 18 ± 5 nm for MB and SY catalysis, respectively (ESI, Fig. S8†).| Dye | Experimental condition MB/Ag/NaBH4 (moles/moles/moles) | k (10−2 s−1) | k2 (10−2 s−1) | k3 (10−2 s−1) |
|---|---|---|---|---|
| MB | 1/12/1700 | 7.65 ± 0.11 | 0.80 ± 0.09 | 0.07 ± 0.01 |
| SY | 1/4/21 | 1.40 ± 0.30 | 0.05 ± 0.02 | 0.02 ± 0.01 |
On the other hand, if we compare XPS analysis on the as-prepared catalyst and on homologues samples after the catalytic tests (ESI, Fig. S2,† spectra A, B, C) no appreciable difference in the silver chemical state took place. Nevertheless, XPS data of AgNPs/(SiO2-PEI-2) after MB catalysis (ESI, Fig. S4†) evidenced the presence of dye residuals. Taken together, these results seem to suggest that the decreasing of kinetic constant could be attributable both to the AgNPs size variation and to a dye absorption before the catalytic reaction (see ESI, Fig. S10†). Further experimental efforts are needed to produce more stable systems endowed with an enhanced life cycle.
4. Conclusions
Commercial silica PEI can be a convenient, cheap substrate/reagent for preparing, in a very straightforward way, supported AgNPs with an average size of 9 ± 2 nm. The overall process leads to a system capable of both reducing the Ag(I) precursor, stabilizing the resulting AgNPs without the need for any additional agent and preventing aggregation. The XPS analysis of the AgNPs/(SiO2-PEI) system reveals that silver was mainly present in its metallic state. The resulting materials can behave like heterogeneous catalysts towards the degradation of Methylene Blue in presence of NaBH4, and show kinetic constants (0.80–7.67 × 10−2 s−1) comparable or higher than those reported in the literature for similar applications. Finally, AgNPs/(SiO2-PEI) can be also successfully employed for decoloration of some azo dyes, such as Sunset Yellow and Azorubine, largely used in food industry. FE-SEM and XPS analyses carried out on AgNPs/(SiO2-PEI) after being used in the catalytic tests showed that an aggregation of Ag nanoparticles occurred and it is responsible for a decrease in the catalytic performances. Further experimental efforts to produce more stable systems endowed with an enhanced life cycles are under way.Acknowledgements
A.M. thanks the Center for Industrial Research - Advanced Applications in Mechanical Engineering and Materials Technology (CIRI-MAM), University of Bologna for financial support. C.M. and D.B. acknowledge the financial support under the FP7 projects “SOLAROGENIX” (NMP4-SL-2012-310333), Padova University ex-60% 2012–2013, PRAT 2010 (no. CPDA102579) and Regione Lombardia-INSTM ATLANTE projects. The authors are grateful to Dr Simone Bugani for XRF analysis and to Dr Carla Boga for azo dyes supply.References
- A. Debnath and S. Chakraborty, Experimental design to optimize color removal of diazo dye Congo Red using zero valent iron, Int. J. Environ. Waste Manage., 2013, 11, 267–288 CAS.
- A. J. Greaves, J. H. Churchley, M. G. Hutchings, D. A. S. Phillips and J. A. Taylor, A chemometric approach to under-standing the bioelimination of anionic, water-soluble dyes by a biomass using empirical and semi-empirical molecular descriptors, Water Res., 2001, 35, 1225–1239 CrossRef CAS.
- Z. Jia, H. Sun, Z. Du and Z. Lei, Catalytic bubble-free hydrogenation reduction of azo dye by porous membranes loaded with palladium nanoparticles, J. Environ. Sci., 2014, 26, 478–482 CrossRef CAS.
- Y. Li, A. H. Lu, S. Jin and C. Q. Wang, Photo-reductive decolorization of an azo dye by natural sphalerite: case study of a new type of visible light-sensitized photocatalyst, J. Hazard. Mater., 2009, 170, 479–486 CrossRef CAS PubMed.
- R. Rajesh, S. S. Kumar and R. Venkatesan, Efficient degradation of azo dyes using Ag and Au nanoparticles stabilized on graphene oxide functionalized with PAMAM dendrimers, New J. Chem., 2014, 38, 1551–1558 RSC.
- J. Li, X. Chen, N. Ai, J. Hao, Q. Chen, S. Strauf and Y. Shi, Silver nanoparticle doped TiO2 nanofiber dye sensitized solar cells, Chem. Phys. Lett., 2011, 514, 141–145 CrossRef CAS PubMed.
- K. Awazu, M. Fujimaki, C. Rockstuhl, J. Tominaga, H. Murakami, Y. Ohki, N. Yoshida and T. Watanabe, A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide, J. Am. Chem. Soc., 2008, 130, 1676–1680 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Silver nanoparticles as a new generation of antimicrobials, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- S. Fazzini, D. Nanni, B. Ballarin, M. C. Cassani, M. Giorgetti, C. Maccato, A. Trapananti, G. Aquilanti and S. I. Ahmed, Straightforward Synthesis of Gold Nanoparticles Supported on Commercial Silica-Polyethyleneimine Beads, J. Phys. Chem. C, 2012, 116, 25434–25443 CAS.
- X. Sun and Y. Luo, Preparation and size control of silver nanoparticles by a thermal method, Mater. Lett., 2005, 59, 3847–3850 CrossRef CAS PubMed.
- V. K. Vidhu and P. Daizy, Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower, Spectrochim. Acta, Part A, 2014, 117, 102–108 CrossRef CAS PubMed.
- ImageJ, http://www.imagej.nih.gov/ij/, accessed, July 2014.
- http://www.srdata.nist.gov/xps.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, ed. J. Chastain, Perkin Elmer Corporation, Eden Prairie, MN, 1992 Search PubMed.
- D. Briggs and M. P. Seah, Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, J. Wiley & Sons, 1983 Search PubMed.
- Z. Lei, L. Zhang and X. Wei, One-step synthesis of silver nanoparticles by sonication or heating using amphiphilic block copolymer as templates, J. Colloid Interface Sci., 2008, 324, 216–219 CrossRef CAS PubMed.
- P. Praus, M. Turicová, M. Karlíková, L. Kvítek and R. Dvorský, Nanocomposite of montmorillonite and silver nanoparticles: characterization and application in catalytic reduction of 4-nitrophenol, Mater. Chem. Phys., 2013, 140, 493–498 CrossRef CAS PubMed.
- V. K. Vidhu and D. Philip, Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flore, Spectrochim. Acta, Part A, 2014, 117, 102–108 CrossRef CAS PubMed.
- V. S. Suvith and D. Philip, Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles, Spectrochim. Acta, Part A, 2014, 118, 526–532 CrossRef CAS PubMed.
- M. M. Khan, J. Lee and M. H. Cho, Au@TiO2 nanocomposites for the catalytic degradation of methyl orange and methylene blue: an electron relay effect, J. Ind. Eng. Chem., 2014, 20, 1584–1590 CAS.
- Z. J. Jiang, C.-Y. Liu and L.-W. Sun, Catalytic properties of silver nanoparticles supported on silica spheres, J. Phys. Chem. B, 2005, 109, 1730–1735 CrossRef CAS PubMed.
- Y. Lu, Y. Mei, M. Ballauff and M. Drechsler, Thermosensitive Core–Shell Particles as Carrier Systems for Metallic Nanoparticles, J. Phys. Chem. B, 2006, 110, 3930–3937 CrossRef CAS PubMed.
- S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, Synthesis and Size-Selective Catalysis by Supported Gold Nanoparticles:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Study on Heterogeneous and Homogeneous Catalytic Process, J. Phys. Chem. C, 2007, 111, 4596–4605 CAS.
Study on Heterogeneous and Homogeneous Catalytic Process, J. Phys. Chem. C, 2007, 111, 4596–4605 CAS. - M. Stylidi, D. I. Kondarides and X. E. Verykios, Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions, Appl. Catal., B, 2003, 40, 271–286 CrossRef CAS.
- M. Snehalatha, C. Ravikumar, I. Hubert Joe, N. Sekar and V. S. Jayakumar, Spectroscopic analysis and DFT calculations of a food additive Carmoisine, Spectrochim. Acta, Part A, 2009, 72, 654–662 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional XRD, FE-SEM, XPS, XE-AES characterizations for the AgNPs/(SiO2-PEI-2) samples, UV-Vis spectra for the catalytic reduction of SY and AZ and a comparison of catalytic data. See DOI: 10.1039/c4ra14069a |
| This journal is © The Royal Society of Chemistry 2015 |