A hybrid catalyst composed of reduced graphene oxide/Cu2S quantum dots as a transparent counter electrode for dye sensitized solar cells†
Abstract
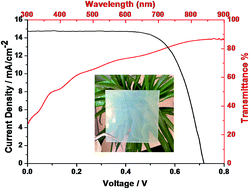
We synthesized a hybrid catalyst of reduced graphene oxide/Cu2S quantum dots (RGO/Cu2S QDs) via a facile wet chemical approach. The synergistic effect between ultrathin-RGO and ultrasmall-QDs endowed the hybrid catalyst with a high transparent performance, excellent conductivity and catalytic activity. A dye-sensitized solar cell fabricated with the hybrid catalyst showed the overall power conversion efficiency was 7.12%, which was comparable to that of a Pt-based device.

 Please wait while we load your content...
Please wait while we load your content...