Structural lipid nanoparticles self-assembled from electrospun core–shell polymeric nanocomposites†
Abstract
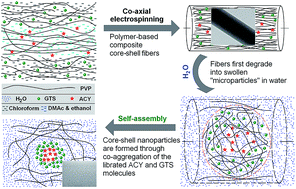
Electrospun polymeric core–shell nanocomposites are exploited as templates to manipulate molecular self-assembly for preparing structural lipid nanoparticles, during which the confinement effect of fibers together with their core–shell structure, the aqueous environment and the secondary interactions, all contributed synergistically to facilitate molecular self-aggregation to produce lipid nanoparticles with a drug entrapment efficiency of 95.9% with a sustained drug release profile.

 Please wait while we load your content...
Please wait while we load your content...