Synthesis, characterization and drug release studies of poly(2-hydroxyethyl methacrylate)/KIT-5 nanocomposite as an innovative organic–inorganic hybrid carrier system
Abstract
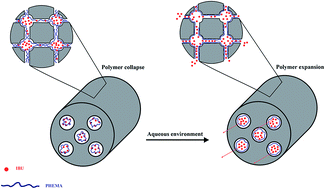
Poly(2-hydroxyethyl methacrylate)/KIT-5 as an ordered mesoporous polymer–silica nanocomposite was synthesized through in situ polymerization. The chemical and physical properties of the nanocomposite were analyzed using a series of different techniques, including XRD, BET, FT-IR, SEM and TEM analysis. Afterward, this innovative ordered mesoporous nanocomposite was utilized for the delivery and controlled release of the model drug ibuprofen. In addition, the in vitro cytotoxicity of the nanocomposite was assessed by MTT assay. Overall, the results were indicative of the potential of the nanocomposite as an excellent carrier system.

 Please wait while we load your content...
Please wait while we load your content...