Quantifying the distribution of ceria nanoparticles in cucumber roots: the influence of labeling†
Abstract
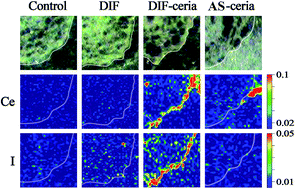
Monitoring the fate of nanoparticles (NPs) in the environment and organisms is always the first step towards better understanding the mechanisms for their toxicity. Fluorescent labeling is one of the most common methods to detect the NPs in samples, however, the attachment of a fluorescent tag to NPs might cause unwanted changes in the distribution and bio-effects of NPs. In the present work, the distribution of ceria nanoparticles (nano-ceria) in cucumber roots after fluorescent labeling, as well as the influence of labeling, was studied. Nano-ceria sized 6.6 nm was labeled by surface coating with diiodofluorescein (DIF), so that the distribution of NPs and the fluorophore could be determined simultaneously by micro-synchrotron radiation X-ray fluorescence (μ-SRXRF) analysis. The two-dimensional mappings of Ce and I in the roots treated with DIF, nano-ceria, and DIF-coated nano-ceria were compared with each other. DIF-coating, though only 25% of the particulate surface was coated, might alter the surface properties of nano-ceria, thereby changing its distribution in cucumber. The co-existence of nano-ceria and DIF greatly enhanced the contact between seeds and DIF, increased the local concentration of DIF on the root surface, and exacerbated the phytotoxicity of DIF. To the best of our knowledge, this is the first study focusing on how the labeling protocol affects the distribution and bio-effects of both the labeling tag and the NPs. Therefore, a guideline on the applicability of the labeling protocol should be developed to ensure the nanotoxicological data obtained using labeling techniques are precise and reliable.

 Please wait while we load your content...
Please wait while we load your content...