Optimization of the theoretical photosynthesis performance and vision-friendly quality of multi-package purplish white LED lighting†
Ji Hye Oh,
Heejoon Kang,
Hoo Keun Park and
Young Rag Do*
Department of chemistry, Kookmin University, 77 Jeongneung-Ro, Seongbuk-Gu, Seoul, 136-702, Korea. E-mail: yrdo@kookmin.ac.kr
First published on 10th February 2015
Abstract
This study introduces the new figures of merit for photosynthesis and photo-pigmentation performance as well as vision performance for photosynthetically efficient, vision-friendly and tunable greenhouse LED lighting sources. The recently developed figures of merit, i.e., the photosynthetic luminous efficacy of radiation (PLER, plm W−1), the photosynthetic luminous efficacy (PLE, plm W−1), the photosynthesis illuminance (PIL), and the photosynthesis action factor (PAF) are measured and added to the measurements of the widely used figures of merit of vision and color to compare the four-package white LEDs developed by our group with natural sunlight and the presently commercialized artificial lamps for greenhouse or indoor use. In this study, one of the best selections of various four-package white LEDs (λ = 630, 590, 520, and 450 nm) shows excellent photosynthetic performance (PLER = 469 plm W−1, PLE = 169 plm W−1) and good vision performance (LER = 271 lm W−1, LE = 98 lm W−1, CRI = 86) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K correlated color temperature (CCT) of blue-enriched white LED. Furthermore, this four-package white LED provides the tunable capability of the spectral power distribution (SPD) of lighting and excellent photosynthetic performance (PLER > 457 plm W−1, PLE > 157 plm W−1) even at eye-friendly vision performances (LER > 249 lm W−1, LE > 87 lm W−1, CRI > 85) under purplish emitting conditions. It can be expected that the four-package purplish white LEDs based on LPDF-capped, phosphor-converted monochromatic LEDs satisfy the requirements to attain high photosynthetic efficiency for the improved growth of plants, energy savings, vision-friendliness, and tunable figures of merit for the four-package LED lighting in greenhouses.
000 K correlated color temperature (CCT) of blue-enriched white LED. Furthermore, this four-package white LED provides the tunable capability of the spectral power distribution (SPD) of lighting and excellent photosynthetic performance (PLER > 457 plm W−1, PLE > 157 plm W−1) even at eye-friendly vision performances (LER > 249 lm W−1, LE > 87 lm W−1, CRI > 85) under purplish emitting conditions. It can be expected that the four-package purplish white LEDs based on LPDF-capped, phosphor-converted monochromatic LEDs satisfy the requirements to attain high photosynthetic efficiency for the improved growth of plants, energy savings, vision-friendliness, and tunable figures of merit for the four-package LED lighting in greenhouses.
Introduction
The most important sustainable-energy production process associated with light in nature is photosynthesis, in which most plants, algae and some bacteria transform solar light into organic compounds that can be used as energy sources to maintain life. In general, artificial lights are generated for photosynthesis from traditional lighting sources, such as high pressure sodium (HPS) lamps and other metal halides (MH) in greenhouses, which are production facilities for the massive cultivation of vegetables or flowers. Recently, the light-emitting diodes (LEDs) are an attractive solid-state lighting technology for the greenhouse industry compared with the currently commercialized lightings, such as HPS, MH and incandescent lamps due to their distinct advantages of the high energy efficiency for photosynthesis, the low radiant heat output, the easy control of spectral composition and the facile adaptation of light intensity to the plant photoreceptors.1–4 In addition, an LED can be easily integrated with various sensors and digital controllers so as to manipulate smart lighting programs for the photosynthesis of plants such as by varying the emission ratio of blue to red colored LEDs, frequency, and intensity for plant species and growth stages.5 Therefore, over the last two decades, many researchers have made a lot of effort to make LEDs an energy efficient lighting alternative to improve the flowering and photosynthetic efficiency of plants in greenhouses.6–10The impact of LEDs on photosynthesis is closely related to the match level of the spectral power distribution (SPD) of LED lamps with the photosynthesis action spectrum (PAS, P(λ)), which represents the efficiency of each wavelength in inducing photosynthesis for averaged vegetable species.11 However, the PAS (P(λ)) curves differ from one species to another because different plant or microorganism species use their own combination of photoreceptor pigments for photosynthesis. After we compared two of them, we selected the PAS (P(λ)) curve adopted by the German Institute for Standardization, numbered DIN 5031-10, in this study.12 The PAS (P(λ)) curve is directly related to chlorophyll a (CL a) and chlorophyll b (CL b) pigments because they are the most common pigments for photosynthesis in green plants. These two pigments show two high sensitivity peaks, one in the blue (B) region at ∼450 nm and the other in the red (R) part of the spectrum at ∼660 nm.12,13 Therefore, the excellent match in the blue and red wavelengths of the PAS (P(λ)) curve and the emission spectrum of combined blue and deep-red LED light sources promotes photosynthesis in most green plants under these lighting environments. Moreover, additional colored LEDs, which emit at different wavelengths, can be easily added to enhance photosynthetic efficiency.
It is known that the range of wavelengths that most plants can use for photosynthesis has evolved over a very long time, in order to effectively use the broad spectrum of sunlight. To date, most researchers have used narrow-band B and R semiconductor LEDs for the application of LEDs in horticulture.14–17 As previously reported, different semiconductor material systems, such as InGaN for blue LEDs and AlGaInP for red LEDs, behave very differently with respect to the surrounding temperature.18 In addition, the low efficiency of semiconductor green and amber LEDs (green gap or yellow gap) limits the application of these colored LEDs in greenhouse lighting. To date, there are a few studies that investigate the suitability of other colored LEDs, except for blue, red, and white LEDs, for a dedicated light source for the optimal photosynthesis of plants in greenhouses. To solve these problems, there is one possible solution based on the use of phosphor-converted LED (pc-LED), which converts blue into any color in the wavelength rage between green and near IR. The selection of color-by-blue approach is due to the existence of an ultra-efficient and stable InGaN blue LED, the development of an excellent blue-mirror-yellow-window dichroic filter, and many high efficiency green, amber, red, and deep-red phosphors, which are excited by blue. The choice of good phosphors in the pc-LED provides a variety of colored lights, tunable emission of photoluminescence (PL) spectrum between 500 and 670 nm, improved efficiency of green and amber LEDs in the “green gap” wavelength ranges, and improved temperature stability of amber to deep-red range of pc-LEDs, compared with less temperature stable semiconductor amber- and red-emitting LED.18 However, there is no concerted approach to controlling the SPDs of colored pc-LED lamps using the unique spectral tunability of various phosphor materials to optimize their effects on the efficiency of photosynthesis. Therefore, it is necessary to consider the figures of merit to estimate and control the potential impact of the SPD of monochromatic pc-LEDs or a combined multi-package of colored pc-LEDs and a blue LED on the photosynthesis action spectrum (PAS (P(λ)) and photo-pigment sensitivity action spectrum (PpAS (Pp(λ)) curve of photosynthesis. Herein, p denotes the photo-pigment in the photoreceptor of plants.
In this study, first, we demonstrate the meaningful figures of merit required for explaining the photosynthesis efficiency and photo-pigment action efficiency of light sources. Using the same concept of defining luminous efficacy of radiation (LER, lm W−1) for human vision, both the photosynthetic luminous efficacy of radiation (PLER, plm W−1) and photo-pigmented luminous efficacy of radiation (PpLER, pplm W−1) are suggested to explain how photosynthetically bright the radiation of the emission spectrum are as perceived by the photoreceptor systems of average plants and each of the pigment in different plant species. Recently, we created a series of RB,M, AB,M, and GB,M monochromatic LEDs, which represents long wavelength pass dichroic filter (LPDF)-capped, monochromatic red, amber, and green pc-LEDs pumped by a blue LED chip to enhance the luminous efficacy of green gap LEDs, improve the temperature stability and control the peak wavelength and bandwidth of pc-LEDs.18–21 B denotes an InGaN blue LED. Herein, using our monochromatic pc-LED concept, we fabricate LPDF-capped green, amber, red, and deep-red full down-converted monochromatic pc-LEDs using various phosphors. Next, we characterize the photosynthetic and visual optical properties of LPDF-capped, monochromatic pc-LEDs as well as the semiconductor type of monochromatic LEDs to determine their suitability for the application in photosynthetic reactions induced by specific photo-pigments in plants. Finally, we demonstrate the photosynthetic optical properties of sunlight during daytime, commercialized artificial lighting sources and multi-package pc-LED systems that consist of two, or four combination of RB,M, AB,M, and GB,M pc-LEDs, and a blue LED in an effort to propose the way to find the higher PLER, PLE, PpLER, and PpLE of multi-package pc-LEDs. In detail, we also dynamically control the ratio and shape of reddish and bluish emissions of both RB two-package and RB,MAB,MGB,MB four-package pc-LEDs with a change of emission wavelength of red pc-LEDs without sacrificing the photosynthetic energy efficiency to evaluate the feasibility of multi-package pc-LEDs as an excellent photosynthetic lighting sources, with vision-friendly color, as well as smart and tunable greenhouse lighting sources.
Experimental
Fabrication of partially-converted cyan pc-LEDs18–21
To fabricate partially-converted cyan pc-LEDs, an InGaN blue LED (λmax = 445 nm) was used as an excitation source for the various color phosphors of pc-LEDs. The blue LED chips were purchased from Dongbu LED, Inc. The cyan phosphor ((Ba,Sr)2SiO4:Eu) was purchased from Merck Co. 10–50 wt% of cyan phosphor ((Ba,Sr)2SiO4:Eu) were dispersed in a silicone binder to create a phosphor paste, and the same amount of resulting phosphor pastes were dropped onto a cup-type blue LED. After dropping the phosphor paste onto a blue LED, the paste was hardened by heating in each case. The partially-converted cyan pc-LEDs were named with its color and wt% (e.g., 10 wt% partially-converted cyan pc-LED: C10 wt%).Fabrication of LPDF-capped monochromatic red/amber/yellow/green pc-LEDs18–21
To fabricate LPDF-capped monochromatic red/amber/yellow/green pc-LEDs, an InGaN blue LED (λmax = 445 nm) was used as an excitation source for the various color phosphors of pc-LEDs. The fabrication method of the LPDF is in the ESI experimental section.† The blue LED chips were purchased from Dongbu LED, Inc. The green/yellow phosphors were purchased from Merck Co. and amber/red phosphors were purchased from Intematix cooperation. The narrow-band red emitting phosphor, K2SiF6:Mn, was synthesized from our laboratory (see experimental in the ESI†).22 Optimum amounts of each color phosphor were dispersed in a silicone binder to create a phosphor paste, and the same amounts of resulting phosphor pastes were dropped onto a cup-type blue LED to create each colored pc-LED. After dropping the phosphor paste onto a blue LED, the paste was hardened by heating. The LPDFs (L535 for yellow/green and L550 for red/amber) were capped on the top of the pc-LEDs with an air gap to realize full down-converted pc-LEDs to realize monochromatic color. The green/amber/red semiconductor-type LEDs were referred to according to their colors (e.g., red LED: R630S and R660S, amber LED: A590S, and green LED: G517S); the blue LED was named based on its color and wavelength (B450S); and the pc-LEDs were named based on their color and wavelength (e.g., a LPDF-capped green pc-LED with an emitting wavelength of 520 nm: G520).Characterization of partially converted cyan pc-LEDs, LPDF-capped red/amber/yellow/green pc-LEDs, and four-package white LEDs
The emission spectra and luminous flux of the forward emission from cyan pc-LEDs, LPDF-capped red/amber/yellow/green pc-LEDs, and red/amber/green/blue semiconductor-type LEDs were measured in an integrated sphere using a spectrophotometer (PSI Co. Ltd., Darsapro-5000) with an applied current of 60 mA (rated current). The emission spectra and luminous flux of the two- and four-package LED systems were measured in an integrated sphere using a spectrophotometer (PSI Co. Ltd., Darsapro-5000) by controlling the applied current of each primary LED with a total applied current of 240 mA. A set of four-package pc-LEDs (R630, A590, G520, B450S) was selected for the reference primary LEDs to compare the optical properties with a set of four CCTs (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K, 6500 K, 3500 K, and 2000 K) by changing each colored pc-LED.
000 K, 6500 K, 3500 K, and 2000 K) by changing each colored pc-LED.
Results and discussion
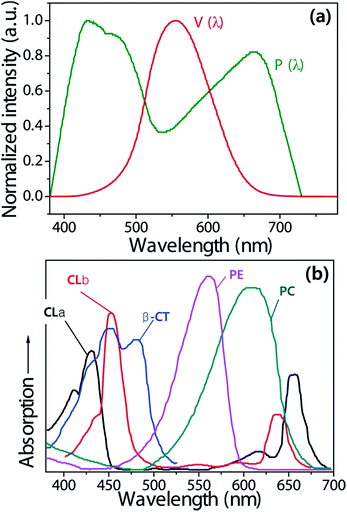
As shown in the PAS (P(λ)) and PpAS (Pp(λ)) curve in Fig. 1a and b, chlorophylls (CL a and CL b) play an important role in photosynthesis and strongly absorb the blue and red wavelengths of light.23 However, plants have other antenna pigments, such as the carotenoids β-carotene (β-CT), phycoerythrin (PE), and phycocyanin (PC), to absorb different colors from both blue and red light for supporting the growth of plants (Fig. 1b). In addition, several reports have shown that the growth, flowering, and metabolism of plant species is specific to the color of light absorbed. It is also reported that green light can contribute to the plant growth and development.24–26To select the proper SPD of phosphors and monochromatic pc-LEDs, the effects of the light quality of colored pc-LEDs on the photosensitivity curves of all photo-pigments, contributing to the photosynthesis of plants should be considered. Nearly all of the photosynthetic performance indicators of monochromatic pc-LED and LED light, such as the photosynthesis and photo-pigment luminous efficacy and illuminance, can be calculated on the basis of P(λ) and Pp(λ), using the same concept as the calculation of figures of merit of the vision performance of artificial lightings based on photopic sensitivity action curve (V(λ)).27,28 Similarly, the photo-pigment action efficiency of each pc-LED can be also calculated on the basis of the sensitivity curve of all the photo-pigments, as shown in Fig. 1b.
Accordingly, the photosynthetic luminous efficacy of radiation (PLER, Kp) and photo-pigmented luminous efficacy of radiation (PpLER, Kpp) can be defined as the ratio of the photosynthesis luminous flux to the radiant flux (S(λ)) and photo-pigment luminous flux to the radiant flux (S(λ)), respectively, using the same concept to calculate the luminous efficacy of radiation (LER), in lumens per watt (lm W−1), which is a parameter explaining how visually bright the radiation of the emission spectrum is as perceived by the average human eye (see eqn (1) and (2)).29
 | (1) |
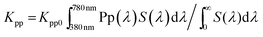 | (2) |
| PAF (plm lm−1) = PLER (plm W−1)/LER (lm W−1) | (3) |
| PpAF (pplm lm−1) = PpLER (pplm W−1)/LER (lm W−1) | (4) |
To assess the vision and photosynthesis performance of actual monochromatic LEDs, multi-package LEDs, and general artificial lighting systems, several figures of merit should also be considered. The first important figure of merit for the visual energy efficiency of real lighting sources is the luminous efficacy, as measured in lumens per watt (lm W−1). In addition, the external quantum efficiency (EQE, ηe) of each lighting system is defined as the ratio of the luminous efficacy (LE, ηlm) to the LER. With the same logical sense, the photosynthesis and photo-pigmented luminous efficacy (PLE, ηp, and PpLE, ηpp) are defined by multiplying the PLER and EQE (and the PpLER and EQE) of each lighting source, respectively (see eqn (5)–(7)).21
| ηe = ηlm/LER, | (5) |
| ηp = PLER × ηe | (6) |
| ηpp = PpLER × ηe | (7) |
It is possible to evaluate the monochromatic LEDs, multi-package LEDs, and pc-LEDs lighting of alternative light sources by acquiring spectral distributions and photosynthetic (photo-pigmented) luminance per watt (plm W−1 (or pplm W−1)) and computing the corresponding figures of merit, in this case the photosynthesis luminous efficacy and photo-pigmented luminous efficacy.
In order to determine the minimum amount of light from individual artificial light sources to improve the photosynthetic efficiency of greenhouse systems, it is also important to measure the photosynthetic (or photo-pigmented) illuminance (PIL (or PpIL)) of the light source instead of relying on the visual illuminance (VIL) value. Similar to the illuminance measurement method, the PIL (or PpIL) can be obtained by measuring the total photosynthetic (or photo-pigmented) luminous flux incident on a surface per unit area. The only different concept when measuring the PIL (or PpIL) from the VIL is the use of the photosynthesis (or photo-pigment) action spectrum (P(λ) (or Pp(λ))) instead of the CIE photopic action curve (V(λ)) while calculating the photosynthesis luminous flux.30 Simply, the PIL and PpIL can be defined from the equation below:
| PIL (plm m−2, plx) = PAF (plm lm−1) × VIL (lm m−2, lx) | (8) |
| PpIL (pplm m−2, pmlx) = PpAF (pplm lm−1) × VIL (lm m−2, lx) | (9) |
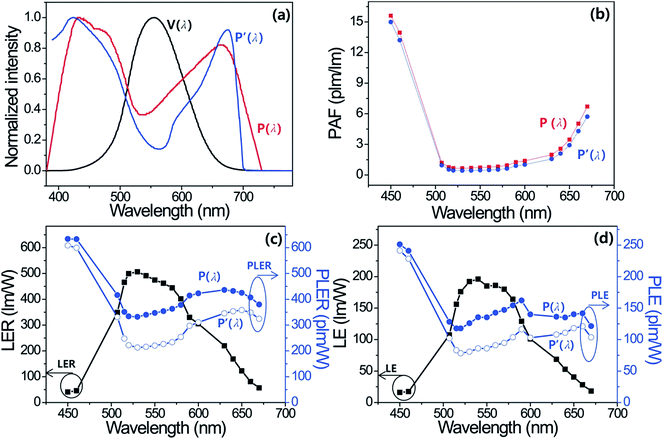
If we know the PAF or PpAF of any type of light source and the VIL, we can calculate the PIL or PpIL of the light source under a specified condition. Consequently, one must bear in mind that a high PAF (or PpAF) and a high PIL (or PpIL) value for individual artificial light sources are achieved under the same VIL conditions. Furthermore, the LED lamp spectra, which can have a high PAF (or PpAF) value, have the potential to concentrate the energy in a spectral region that is photosynthesis-sensitive (or photo-pigment-sensitive), thus having a strong impact on the photosynthesis of plants in greenhouse systems. A variety of efficient monochromatic and multi-package LEDs were characterized to determine the photosynthetic performances as well as the vision performances of their pc-LEDs. Fig. S1a–S1f† show the normalized photoluminescence (PL) spectra and color coordinates of six RAGB semiconductor LEDs, five partially converted cyan phosphor-coated blue LEDs, and sixteen RB,M, AB,M, and GB,M LPDF-capped, monochromatic pc-LEDs, which were fabricated by simply capping LPDF on top of the InGaN blue LED with each corresponding phosphor. These figures indicate that the emission spectrum and color coordinates of all the monochromatic pc-LEDs are well matched with those of the corresponding phosphors in our previous publications.21 Herein, all the monochromatic LEDs are denoted as a combination of the color and peak wavelength of the emission spectrum. In addition, S denotes the semiconductor-type of monochromatic LEDs. Moreover, partially converted cyan LEDs denote color and the weight% of phosphor in paste. All LERs, LEs, PLERs, PpLERs, PLEs, PpLEs, and PAFs of monochromatic colored LEDs for both the visual performance of eye and photosynthesis performance of plants are measured at 60 mA (rated current) and displayed with the change of the peak wavelength of the monochromatic pc-LEDs and semiconductor LEDs in Tables S1a and S1b in the ESI.† Unfortunately, to date, there are several different photosynthesis action spectra (P(λ)) to evaluate the photosynthetic performance in the different species, for example: Aubé et al.,11 and Purves et al.,13 proposed different P(λ)s, depending on the plant species and measuring conditions. Among these, we compared the different types of photosynthesis action spectra (P(λ)) from Aubé et al. (P(λ)), and Purves et al., (P′(λ)). Fig. 2a and b show the photosynthesis action spectra and PAF of each blue LED and cyan/green/amber/red LPDF-capped pc-LEDs from 450 nm to 670 nm. In addition, we compare the PLERs and PLEs of monochromatic LEDs with different wavelengths of EL emission. As shown in Fig. 2b–d, both PAFs have similar values regardless the photosynthesis action spectra, but PLER and PLE calculated from P(λ) are higher than those from P′(λ), owing to the higher sensitivity of P(λ) over the wavelength ranges from 430 to 600 nm. Therefore, we used the photosynthesis action spectra (P(λ)) from the German Institute for Standardization, numbered DIN 5031-10 in this experiment.11,12 Herein, the figures confirm that the shapes of the dependent graphs of PLER and PLE on the peak wavelength are identical in appearance to the photosynthesis action spectra (PAS; P(λ) and P′(λ)). The figures also indicate that bluish and reddish LEDs have higher PAF values (the ratio of PLER to LER) and that the greenish LEDs have lower PAF values as is expected. This indicates that bluish and reddish LEDs are more effective than the greenish LEDs on the photosynthesis system of plants. Fig. S2a and S2b† show that the trends of PpLERs and PpLEs of five different photo-pigments with the peak wavelength resemble the wavelength dependence of the photo-pigment sensitivity action spectrum PpAS (Pp(λ)) of each photo-pigment (see Fig. 1b).
As expected, different photo-pigment has maximum luminous efficacy at different EL wavelength of monochromatic LEDs. These figures show that the bluish LEDs have the highest photo-pigmented efficiency of CL a, CL b and β-CT. Otherwise, the greenish (530 nm) LED and the amber (590 nm) LED have the maximum photo-pigmented efficacy of PE and PC photo-pigment, respectively. Most plants and major pigments for photosynthesis respond strongest to red and blue light for photosynthesis, otherwise, other antenna pigments (such as PE and PC), which participate in light absorption and play a significant role in photosynthesis, respond strongest to green and amber light. Therefore, the use of green and amber LEDs, as well as blue and red LEDs, should be considered to have a balanced effect on the shape, development and flowering (photomorphogenesis) of growing plants. The characteristics of artificial lighting types that are ideal for vision are fairly different from than those that are maximally effective for the photosynthesis system. The most general lighting and greenhouse lighting sources used in the present market were not developed exclusively for improving the photosynthesis performance and photo-pigment performance by considering the match SPDs of light sources and P(λ) and Pp(λ)s of plants. Fig. S3, Tables S2a and S2b† show the reported or measured SPDs and figures of merit of 10 commercialized types of lighting and daylight. These data indicate that the SPDs of all 10 artificial lighting types used presently for general lighting and greenhouse lighting are fixed with a specific correlated color temperature (CCT). Herein, we also measured and calculated the figures of merit of daylight as a standard source for photosynthesis in order to know the requirements for mimicking daylight. As reported in our previous publication,21 the amounts of visual and photosynthesis light from the sun, namely, VIL and PIL (or PpIL), simply increase from early in the morning until 12:30 pm in the afternoon and then simply decrease until sunset. Fig. 4k and l indicate that the PAF values of daylight remain nearly similar with small variations near 1.83–2.05. This indicates that the photosynthesis brightness of daylight is around 1.9 fold higher than the value of the visual brightness of daylight. Because P(λ) covers the blue and red parts of visible light but V(λ) covers only green part of skylight from sun. As shown in the photosynthesis performance data of artificial lightings and skylights in Fig. S3, Tables S2a and S2b,† there is no ideal lamp to meet all of the requirements to attain high photosynthesis performance and good photo-pigment performance, while having a highly positive vision effect. Because both figures of merit, such as visual luminous efficacy and photosynthesis luminous efficacy for artificial lighting sources are in a tradeoff relationship, managing the light of an artificial lighting source to optimize the photosynthesis performance of plants, and vision performance of humans become critical. Therefore, it is clear that acquiring the capability of controlling all the figures of merit of lighting sources for photosynthesis and vision is the most important prerequisite for excellent photosynthesis and smart lighting systems.
To date, various two colored LEDs combining blue and red LED have been widely studied for use in greenhouse lighting to enhance the growth of various vegetables in greenhouses.14–17 Although the conventional R and B two-color approach allows the facile dynamic control of R-to-B ratio points and provides high PLER, this approach has disadvantages, such as the different temperature/current dependence of each semiconductor-type R and B LED (see Fig. S1 and S2 in ESI†), the visually-unfriendly violet color to humans, and reduced light quality due to unbalanced colors for all the processes of photosynthesis. Fig. S4 and S5† indicate that amber and green pc-LEDs have reduced or at least similar variations of the efficacy and color coordinates with the current/temperature compared to the wide variation in the levels of current/temperature stability among red monochromatic III–V LED that do not contain phosphors. It means that the blue-to-red ratio of RB,MB two-color LEDs remain almost constant with a small variations as functions of the applied current and temperature in the ranges of the conditions of use. Herein, we demonstrate RB, RB,MB and AB,MB two package violet LED system that consists of a series of amber and red pc-LEDs and a blue LED (λ = 450 nm) in an effort to compare their all photosynthetic figures of merit with RB two-color light. Fig. 3a–c show the schematic illustration of RB, RB,MB, and AB,MB two-color LEDs. Based on the emission spectrum of each two-color LED (see Fig. 3d) and meaningful figures of merit (the LER, LE, PLE, PLER, PpLER, and PpLE) of all types of RB, RB,MB, and AB,MB two-color LEDs for the photosynthesis applications are calculated and summarized, as displayed in Fig. 5e and f with the change of amber and red LEDs. Although the highest PLE (∼446 plm W−1) is obtained by a combined two-color LED of InGaN blue and AlGaInP red LED, it can be speculated that the combined two color LED of InGaN blue LED and red pc-LED, including narrow-band K2SiF6:Mn4+ phosphor (denoted as RKSF), is appropriate for maintaining stable light quality and showing the high PLE value (∼337 plm W−1). However, blue and red two color LEDs have some limitations on the induction of the photoreactions of some photo-pigments, which have a peak of photo-pigment sensitivity action spectrum (Pp(λ)) at green and yellow wavelength because the match level between LED emission spectrum and Pp(λ) is very low. It needs green light even in small amounts to activate antenna pigment, such as a PE for balanced photosynthesis in the growth of plants, as considering the very low PpLE of red and blue two color LEDs in Fig. S6a and S6b.† Therefore, it can be simply speculated that the wide band SPD of possible greenhouse LEDs must include green emission in order to stimulate the healthy and balanced growth of plants.
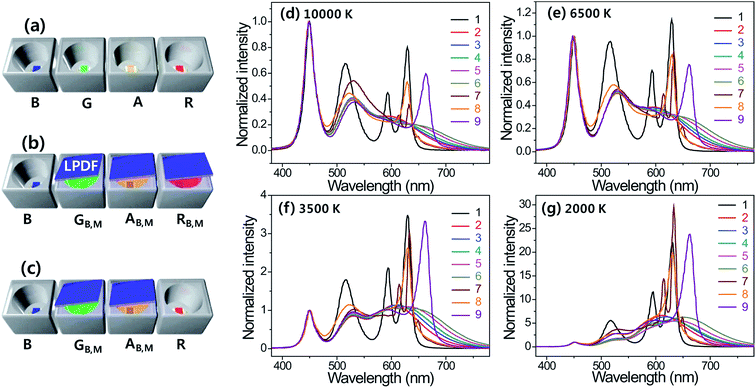
Both the conventional RAGB four-package approach using different colored semiconductor LEDs and the RB,MAB,MGB,MB (RB,MAB,MGB,M representing a LPDF-capped, full down-converted, monochromatic red, amber and green pc-LED pumped by a blue LED chip) approach using full-down converted colored LEDs are possible candidates for the control of SPDs and the intensity of white LEDs. In this experiment, a RAGB multi-package white LED is characterized as a standard sample for comparison. Furthermore, we characterize the figures of merit of various RB,MAB,MGB,MB four-package white LEDs in terms of the vision performance and photosynthesis performance such as LER, LE, CRI, PAF, PLER, and PLE. The schematic illustrations of the RB,MAB,MGB,MB four-package white LEDs in Fig. 4a–c show the assembly of RAGB, RB,MAB,MGB,MB and RAB,MGB,MB four-package LEDs. The total number of combinations of RB,MAB,MGB,MB four-package LEDs using one blue, five cyans, six greens, four amber-yellows, and six reds, provides a great number of different types of white LEDs. Numerous combinations present a lot of four-package LEDs to be assembled and characterized.
Consequently, one four-package RB,MAB,MGB,MB LED is selected as a control white LED in this study. Considering the photo-pigmented luminous efficacies of blue, green and amber LEDs, a set of primary LEDs with peak wavelengths of 590 nm (amber-yellow, A590), 520 nm (green, G520), and 450 nm (blue, B450S) is selected for the RB,MAB,MGB,MB four-package white LEDs by analyzing the effect of varying two narrow-band semiconductor-type LED reds, five broad-band pc-LED reds and on narrow-band pc-LED red on the photosynthesis performance of the four-package white LEDs. Fig. 4d–g show the overlapped integrated emission spectra of each LED in a RAGB, RB,MAB,MGB,MB and RAB,MGB,MB four-package white LED at CCTs of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K, 6500 K (cool white) 3500 K (warm white), and 2000 K (firelight) along with five tunable wide-band red pc-LEDs, one narrow-band red pc-LED and two narrow-band semiconductor red LEDs. The reference of RAGB white LED systems show a narrow spectrum of each colored LED, while the other systems, combined with pc-LEDs, show a broad spectrum at all CCTs.
000 K, 6500 K (cool white) 3500 K (warm white), and 2000 K (firelight) along with five tunable wide-band red pc-LEDs, one narrow-band red pc-LED and two narrow-band semiconductor red LEDs. The reference of RAGB white LED systems show a narrow spectrum of each colored LED, while the other systems, combined with pc-LEDs, show a broad spectrum at all CCTs.
As shown in Fig. 5, the narrow-band RAGB LED and wide-band RAB,MGB,MB or RB,MAB,MGB,MB LED with the narrow-band red LED are superior in terms of the LER and PLER values but the wide-band RB,MAB,MGB,MB four-package LED with the broad-band red LED is superior in terms of the LE and PLE. These figures also indicate that the blue and green portions of the white color decrease, while the red portion of the white color increases with an increase of the red emitting wavelengths at all CCTs. As a result, the LER, LE, PLER, and PLE of the RB,MAB,MGB,MB decrease slowly with an increase of the emitting wavelengths of the red pc-LED at all CCTs in the red series.
This also shows that the PAFs of the RB,MAB,MGB,MB white LED have similar values with an increase in the red emitting wavelengths at one CCT. As is similar to the circadian action factor (CAF),21 the PAF values of the RB,MAB,MGB,MB white LED depend mainly on the variations of the CCTs except for 2000 K. Accordingly, the PAFs of firelight (2000 K) of RB,MAB,MGB,MB LEDs increase from 1.41 to 1.83 with an increase of the emitting wavelength of the red pc-LED. This wide control of PAF values suggests that four-package white LEDs are good artificial lighting candidates that can simultaneously function as lamps for human vision and for the photosynthesis of plants by activating photosynthetic reactions. Furthermore, the PLE values of four-package RB,MAB,MGB,MB and RSAB,MGB,MB white LEDs are higher than those of HPS and MH lamps, which are used for greenhouse lighting. Different from the LE and PLE trends, the color quality properties, i.e., the color rendering index (CRI) of the four-package white-light LED show nearly constant values over 85 with an increase in the emitting wavelengths of the red pc-LED at CCTs of either 6500 or 3500 K and values that exceed 80 at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 or 2000 K (see Fig. 5c).
000 or 2000 K (see Fig. 5c).
This indicates that the four-package white LEDs show good color quality even at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K where they give the highest photosynthesis luminous efficacy. It is well known that the LE and CRI exist in a trade-off relationship in a single-package white pc-LED. Moreover, Fig. 5 indicates that the PLE and CRI values have no direct trade-off relationship in the four-package RB,MAB,MGB,MB and RAB,MGB,MB white LEDs. Among the nine different RB,MAB,MGB,MB and RAB,MGB,MB four-package white LEDs shown in Fig. 5, the highest vision and color performance for human eyes and excellent photosynthetic effects are attained from the different combinations of a red, amber, green, and blue LED in four-package white LEDs. In this figure, a combined four-package LED of R630, A590, G520, and B450S is selected as having the best PLE at CCT of 10
000 K where they give the highest photosynthesis luminous efficacy. It is well known that the LE and CRI exist in a trade-off relationship in a single-package white pc-LED. Moreover, Fig. 5 indicates that the PLE and CRI values have no direct trade-off relationship in the four-package RB,MAB,MGB,MB and RAB,MGB,MB white LEDs. Among the nine different RB,MAB,MGB,MB and RAB,MGB,MB four-package white LEDs shown in Fig. 5, the highest vision and color performance for human eyes and excellent photosynthetic effects are attained from the different combinations of a red, amber, green, and blue LED in four-package white LEDs. In this figure, a combined four-package LED of R630, A590, G520, and B450S is selected as having the best PLE at CCT of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K. This four-package offers excellent color qualities (CRI > 86), excellent vision performances (LER > 295 lm W−1, LE > 105 lm W−1), and a tunable photosynthesis performance (PLER = 451, 425 plm W−1, PLE = 162, 149 plm W−1, PAF = 1.53, 1.34) at cool- and warm-white CCTs of 6500 and 3500 K. In addition, blue-enriched emission (10
000 K. This four-package offers excellent color qualities (CRI > 86), excellent vision performances (LER > 295 lm W−1, LE > 105 lm W−1), and a tunable photosynthesis performance (PLER = 451, 425 plm W−1, PLE = 162, 149 plm W−1, PAF = 1.53, 1.34) at cool- and warm-white CCTs of 6500 and 3500 K. In addition, blue-enriched emission (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K) of RB,MAB,MGB,MB (R630, A590, G520, and B450S) LEDs provide reasonable color quality (CRI = 86), good vision performances (LER = 271 lm W−1, LE = 98 lm W−1) and highly efficient photosynthesis effect (PLER = 469 plm W−1, PLE = 169 plm W−1, PAF = 1.73). Consequently, it can be simply considered that it is not a difficult step to select a good combination of four differently colored LEDs to attain high LE, CRI, and tunable PLER, PLE and PAF values with a variation of the CCT value in the RB,MAB,MGB,MB white-light system. Therefore, RB,MAB,MGB,MB four-package LEDs can be considered as possible artificial lighting candidates to mimic daytime sunlight, reduce the green portion of white light in greenhouses, and maintain good vision performance, good color qualities and controllable photosynthesis effects.
000 K) of RB,MAB,MGB,MB (R630, A590, G520, and B450S) LEDs provide reasonable color quality (CRI = 86), good vision performances (LER = 271 lm W−1, LE = 98 lm W−1) and highly efficient photosynthesis effect (PLER = 469 plm W−1, PLE = 169 plm W−1, PAF = 1.73). Consequently, it can be simply considered that it is not a difficult step to select a good combination of four differently colored LEDs to attain high LE, CRI, and tunable PLER, PLE and PAF values with a variation of the CCT value in the RB,MAB,MGB,MB white-light system. Therefore, RB,MAB,MGB,MB four-package LEDs can be considered as possible artificial lighting candidates to mimic daytime sunlight, reduce the green portion of white light in greenhouses, and maintain good vision performance, good color qualities and controllable photosynthesis effects.
Although the two color LED system of InGaN blue and red pc-LED shows a much higher PLE (∼320 plm W−1) than that of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K RB,MAB,MGB,MB (R630, A590, G520, and B450S) LEDs (∼169 plm W−1), the absence of green and amber color is detrimental to the balanced growth of plants and the visual comfort of workers in greenhouses. The required level of green and amber photons for optimum plant growth is different for different plant species. Our four-package LED lights with tunable and different wavelengths of red, amber, green, and red four colors would be beneficial in determining the species specific optimal wavelengths for plant growth. To study the photosynthesis luminous efficacy of four package LEDs with the change of SPD of RB,MAB,MGB,MB LED lamps, we decrease the ratio of green/amber portion from the ratio value of 10
000 K RB,MAB,MGB,MB (R630, A590, G520, and B450S) LEDs (∼169 plm W−1), the absence of green and amber color is detrimental to the balanced growth of plants and the visual comfort of workers in greenhouses. The required level of green and amber photons for optimum plant growth is different for different plant species. Our four-package LED lights with tunable and different wavelengths of red, amber, green, and red four colors would be beneficial in determining the species specific optimal wavelengths for plant growth. To study the photosynthesis luminous efficacy of four package LEDs with the change of SPD of RB,MAB,MGB,MB LED lamps, we decrease the ratio of green/amber portion from the ratio value of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K white (blue; 75 mA, green; 105 mA, amber; 30 mA and red; 32 mA) to the enriched blue/red portion with the constant blue/red applied current (blue; 75 mA, red; 32 mA).
000 K white (blue; 75 mA, green; 105 mA, amber; 30 mA and red; 32 mA) to the enriched blue/red portion with the constant blue/red applied current (blue; 75 mA, red; 32 mA).
Fig. 6a shows the change of the overlapped integrated emission spectra of RB,MAB,MGB,MB four-package with decrease of the green/amber portion. As shown in Fig. 6a inset, the whitish emission is changed to purplish color when the blue and red portions of emitting spectrum increase with the decrease of green and amber portions. Therefore, the CRI value of the RB,MAB,MGB,MB four-package comes down to zero from 80 with the 0.5 green/amber portion (see Fig. 6b). Fig. 6c and d also show that the PLER and PLE of photosynthesis for plants increase from 469 plm W−1 to 577 plm W−1, and 169 plm W−1 to 226 plm W−1, respectively but the LER and LE of human vision decrease from 271 lm W−1 to 89 lm W−1, and 98 lm W−1 to 35 lm W−1, respectively, with the increase of blue and red portions. As expected, the optimum SPD of the RB,MAB,MGB,MB four-package purplish LED for photosynthesis efficiency is totally reversed from the optimum SPD of whitish LED for efficiency of human vision. Therefore, the spectrum distributions of a RB,MAB,MGB,MB four-package LED should be controlled by the presence or absence of workers in the greenhouse for balancing and maximizing the photosynthesis efficiency of plants and the cultivating and managing efficiency of workers.
Conclusions
The technological advancements for developing good greenhouse LED lighting sources have about a two-decade history since a single-package white pc-LED was first commercialized by Nichia Co. Ltd. To date, all current reports that study the development and optimization of blue and red two color and/or white pc-LED systems have focused on the productivity of plants. The figures of merit, which are widely used in the LED lighting society, are the LER and the LE for vision performance as well as the CRI and CCT for color quality. However, these properties are insufficient when seeking to represent all performances required when searching for excellent artificial lighting sources for greenhouse lighting. Herein, the possible figures of merit are proposed and explained to describe the photosynthesis performance and photo-pigment performance. As explained above, the PLER, PLE, PAF, PpLER, PpLE, and PIL are added to the visual figures of merit of lighting, in this case the PLER, PLE, and PIL values, in order to assess the quality of commercialized artificial lighting sources along with our four-package RB,MAB,MGB,MB LED lighting source and standardize the sunlight with respect to the vision performance and photosynthesis performance. This analysis of the optical data of sunlight, MH lamps, and HPS lamps can provide newly developed artificial greenhouse lighting sources with guidelines for attaining photosynthetically efficient, vision-friendly, and tunable multi-package LEDs by comparing the optical properties of the photosynthesis performance and vision performance among our four-package RB,MAB,MGB,MB LEDs, natural skylight, and presently commercialized light sources. An additional important characteristic for optimizing all the figures of merit for good greenhouse LED lightings is the capability of individual colors to control and adjust the SPDs of LED lighting sources to the optimum SPD for the photosynthesis of plants. The distinct color control of RB,MAB,MGB,MB LEDs combined with a narrow InGaN blue LED and three wide-band LPDF-capped green, amber, red pc-LEDs provides the capability to create the tunable figures of merit while also ensuring excellent photosynthesis performance and good vision qualities. In this study, one of the best choices of four-package RB,MAB,MGB,MB LED (R630, A590, G520, and B450S) shows excellent photosynthesis performance (PLER = 469 plm W−1, PLE = 169 plm W−1) and good vision performance (LER = 271 lm W−1, LE = 98 lm W−1, CRI = 85) at CCT of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K. Furthermore, this RB,MAB,MGB,MB LED provides tunable capability of SPD of lighting and excellent photosynthesis performance (PLER > 457 plm W−1, PLE > 157 plm W−1) even at eye-friendly vision performances (LER > 249 lm W−1, LE > 87 lm W−1, CRI > 85) under blue- and red-enriched purplish emitting conditions. More elaborate experiments are required to realize a photosynthetically efficient, vision-friendly, and tunable multi-package LED lighting system with higher photosynthetic performance, in which the quantum efficiency of the InGaN blue LED chip and phosphors are enhanced and the combination of each colored pc-LED is properly selected in a multi-package greenhouse LED system. This study defines the important figures of merits for efficient photosynthesis lighting systems, which compares the optical properties of natural skylights and commercialized greenhouse lamps, two-package (or two-chip) RB LED lamps, and utilizes four-package RB,MAB,MGB,MB systems with LPDF-capped pc-LEDs, can lead to the creation of high-quality smart lighting systems for high efficiency of photosynthesis, energy savings, low heat generation, good thermal stability, and the realization of vision-friendly purplish white color.
000 K. Furthermore, this RB,MAB,MGB,MB LED provides tunable capability of SPD of lighting and excellent photosynthesis performance (PLER > 457 plm W−1, PLE > 157 plm W−1) even at eye-friendly vision performances (LER > 249 lm W−1, LE > 87 lm W−1, CRI > 85) under blue- and red-enriched purplish emitting conditions. More elaborate experiments are required to realize a photosynthetically efficient, vision-friendly, and tunable multi-package LED lighting system with higher photosynthetic performance, in which the quantum efficiency of the InGaN blue LED chip and phosphors are enhanced and the combination of each colored pc-LED is properly selected in a multi-package greenhouse LED system. This study defines the important figures of merits for efficient photosynthesis lighting systems, which compares the optical properties of natural skylights and commercialized greenhouse lamps, two-package (or two-chip) RB LED lamps, and utilizes four-package RB,MAB,MGB,MB systems with LPDF-capped pc-LEDs, can lead to the creation of high-quality smart lighting systems for high efficiency of photosynthesis, energy savings, low heat generation, good thermal stability, and the realization of vision-friendly purplish white color.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2011-0017449).Notes and references
- G. D. Massa, H.-H. Kim, R. M. Wheeler and C. A. Mitchell, Hortic. Sci., 2008, 43, 1951 Search PubMed.
- D. Singh, C. Basu, M. Meinhardt-Wollweber and B. Roth, http://arxiv.org/ftp/arxiv/papers/1406/1406.3016.pdf.
- C. A. Mitchell, A. Both, C. M. Bourget, C. Kuboto, R. G. Lopez, R. C. Morrow and S. Runkle, Chron. Horticult., 2012, 55, 6–12 Search PubMed.
- R. C. Morrow, Hortic. Sci., 2008, 43, 1947–1950 Search PubMed.
- N. Yeh and J. P. Chung, Renewable Sustainable Energy Rev., 2009, 13, 2175–2180 CrossRef CAS PubMed.
- R. J. Bula, R. C. Morrow, T. W. Tibbits, R. W. Barta, R. W. Ingatius and T. S. Martin, Hortic. Sci., 1991, 26, 203–205 CAS.
- Y. Tanaka, K. Kimata and H. Aiba, EMBO J., 2000, 19, 5344–5352 CrossRef CAS PubMed.
- B. C. Tripathy and C. S. Brown, Plant Physiol., 1995, 107, 407–411 CAS.
- T. Yanagi and K. Okamoto, Acta Hortic., 1997, 418, 223–228 Search PubMed.
- M. Olle and A. Viršile, Agric. Food Sci., 2013, 22, 223–234 Search PubMed.
- M. Aubé, J. Roby and M. Kocifaj, PLoS One, 2013, 8, e67798 Search PubMed.
- DIN 5031-10, German National Standard, 2013.
- W. K. Purves, G. H. Orians and H. C. R. Heller, Life: The Science of Biology, 4th edn, 1994 Search PubMed.
- L. Poulet, G. D. Massa, R. C. Morrow, C. M. Bourget, R. M. Wheeler and C. A. Mitchell, Life Sci. Space Res., 2014, 2, 43–53 CrossRef PubMed.
- G. W. Stutte, S. Edney and T. Skerritt, Hortic. Sci., 2009, 44, 70–82 Search PubMed.
- I. Tarakanov, O. Yakovleva, I. Konovalova, G. Paliutina and A. Anisimov, Acta Hortic., 2012, 956, 171–178 Search PubMed.
- N. Lu, T. Maruo, M. Johkan, M. Hohjo, S. Tsukakoshi, Y. Ito, T. Ichimura and Y. Shinohara, Environ. Control Biol., 2012, 50, 63–74 CrossRef CAS.
- J. H. Oh, J. R. Oh, H. K. Park, Y.-G. Sung and Y. R. Do, Opt. Express, 2011, 19, A270–A279 CrossRef PubMed.
- J. R. Oh, S.-H. Cho, J. H. Oh, Y.-K. Kim, Y.-H. Lee, W. Kim and Y. R. Do, Opt. Express, 2011, 19, 4188–4198 CrossRef CAS PubMed.
- J. H. Oh, S. J. Yang, Y.-G. Sung and Y. R. Do, Opt. Express, 2012, 20, 20276–20285 CrossRef PubMed.
- J. H. Oh, S. J. Yang and Y. R. Do, Light: Sci. Appl., 2014, 3, e141 CrossRef CAS.
- C. Liao, R. Cao, Z. Ma, Y. Li, G. Dong, K. N. Sharafudeen and J. Qiu, J. Am. Ceram. Soc., 2013, 96(11), 3552–3556 CrossRef CAS PubMed.
- M. Johkan, K. Shoji, F. Goto, S. Hahida and T. Yoshihara, Environ. Exp. Bot., 2012, 75, 128–133 CrossRef CAS PubMed.
- K. M. Folta, Plant Physiol., 2004, 135, 1407–1416 CrossRef CAS PubMed.
- H. H. Kim, G. D. Goins, R. M. Wheeler and J. C. Sager, Hortic. Sci., 2004, 39, 1617–1622 Search PubMed.
- A. Novičkovas, A. Brazaitytė, P. Duchovskis, J. Jankauskienė, G. Samuolienė, A. Viršilė, R. Sirtautas, Z. Bliznikas and A. Žukauskas, Acta Hortic., 2012, 927, 723–730 Search PubMed.
- A. Žukauskas, R. Vaicekauskas and P. Vitta, Appl. Opt., 2012, 51, 8423–8432 CrossRef PubMed.
- D. Gall and K. Bieske, Proceedings of the CIE Symposium 2004 on Light and Health: Non-Visual Effects, CIE, Vienna, Austria, Wien, 2004, pp. 129–132 Search PubMed.
- P. F. Smet, A. B. Parmentier and D. Poelman, J. Electrochem. Soc., 2011, 158, R37–R54 CrossRef CAS PubMed.
- D. Lang, Proc. SPIE, 2011, 7954, 795402 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13853h |
| This journal is © The Royal Society of Chemistry 2015 |