A modular approach to self-oscillating polymer systems driven by the Belousov–Zhabotinsky reaction
Abstract
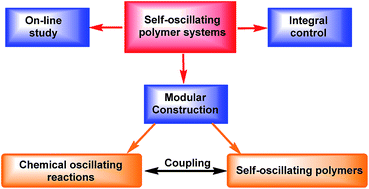
Inspired by the self-oscillating phenomena in nature, such as heartbeat, brain waves, pulsatile secretion of hormones, cell cycles and biorhythms, a new kind of responsive polymers, so-called the self-oscillating polymers (SOPs) are developed. Different from the traditional responsive polymers, SOPs are driven by chemical oscillating reactions and exhibit autonomous response without “ON/OFF” switching of external stimuli. From the perspective of modular design, we explore the principle, construction and integral control of SOP systems based on the recent developments. Attention is also paid to the emergent techniques for on-line study of self-oscillating behaviors and engineering approaches to novel SOP systems with unique functions and high oscillating performances.

 Please wait while we load your content...
Please wait while we load your content...