Nano-hydroxyapatite promotes self-assembly of honeycomb pores in poly(L-lactide) films through breath-figure method and MC3T3-E1 cell functions†
X. H. Wua,
Z. Y. Wub,
J. C. Su*c,
Y. G. Yand,
B. Q. Yuc,
J. Weib and
L. M. Zhao*b
aDepartment of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106, USA
bState Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, P.R. China
cDepartment of Orthopaedics, Changhai Hospital, Second Military Medical University, Shanghai 200433, P.R. China. E-mail: biomaterbone@163.com; zhaoliming@ecust.edu.cn
dCollege of Physical Science and Technology, Sichuan University, Chengdu 610041, P.R. China
First published on 17th December 2014
Abstract
Recently, honeycomb-patterned polymeric films have been fabricated through breath-figure method to mimic the topographical feature of the basement membrane and this topography was demonstrated to enhance osteoblast functions including adhesion, spreading, proliferation, and differentiation. However, honeycomb-patterned films incorporated with other components benefiting osteoblast functions have not been reported yet. In this study, we report the fabrication of nano-hydroxyapatite (nHA) (3 wt%, 5 wt%, and 7 wt%) incorporated honeycomb poly(L-lactide) (PLLA) films to evaluate the effect of nHA on the self-assembly of honeycomb pores in PLLA films. Scanning electron microscopy (SEM) images demonstrated that 5 wt% nHA in PLLA resulted in the most regular honeycomb pores. Water contact angle test and protein adsorptions including fibronectin (FN) and serum were performed on 5 wt% nHA incorporated honeycomb PLLA films (H-PLLA/nHA), as well as the controls, flat PLLA films (F-PLLA), honeycomb PLLA films (H-PLLA), and flat HA films (F-HA). Honeycomb pores in H-PLLA films enhanced hydrophobicity compared with F-PLLA films, whereas nHA particles in H-PLLA/nHA films lowered the hydrophobicity compared with H-PLLA. Both the FN and serum protein adsorptions were increased on H-PLLA films compared with F-PLLA, but decreased significantly on H-PLLA/nHA films compared with H-PLLA films. Further, MC3T3-E1 mouse newborn calvaria preosteoblasts were employed to investigate the cell functions in terms of cell adhesion, spreading, proliferation, and differentiation on those films. The results strongly indicated that those cell functions were promoted on honeycomb films, especially H-PLLA/nHA films, compared with F-PLLA films and they did not show a significant difference between H-PLLA films and F-HA films.
Introduction
Cells react and respond to substrate surfaces when exposed to culture medium via detecting the surface chemistry, topography, and mechanical properties, which govern subsequent events, including cell adhesion, spreading, proliferation, and differentiation.1–3 Hence, engineered biomaterials for tissue regeneration, especially anchorage-dependent cells, have to possess certain advantages in terms of these factors. Among these factors, a special topography, honeycomb pores, has drawn increasing attention due to the fact that honeycomb pores could modulate cell functions. However, the manners in which honeycomb pores influence cell functions are still in debate and are cell type dependent. In some studies, cell functions were promoted by honeycomb pores. Honeycomb polycaprolactone (PCL) films with pores ranging from 3 to 20 μm enhanced adhesion and proliferation of epidermal keratinocytes and dermal fibroblasts and the smaller pores of 3–5 μm supported the best cell functions.4 Also, honeycomb PCL films with a pore size of 1 μm or 4 μm promoted fibroblasts viability compared with flat films.5 Similarly, honeycomb PCL or photo-cured PCL triacrylate films strongly enhanced MC3T3-E1 cell functions including adhesion, attachment, proliferation, and differentiation.6,7 On the other hand, honeycomb pores can suppress cell functions. Honeycomb poly(lactic acid) (PLA) films prohibited chondrocyte proliferation and the differentiation of neural stem cell was suppressed on honeycomb PCL films compared with flat ones.8,9However, recent investigations regarding cell response to honeycomb films have been focused on pure materials, such as PCL and PLA. To date, the studies on honeycomb films incorporating growth factors benefiting cell functions have not been reported. PLLA, as an aliphatic polyester and also a biodegradable polymer, has been extensively investigated to serve as good candidate for tissue regeneration because of its biocompatibility, biodegradability, and low immunogenicity.10–12 Honeycomb PLLA films have also been developed using breath-figure method without assistance of surfactants, however, with more irregular pore dimension compared with other honeycomb films.13,14 During formation of honeycomb pores in breath-figure method, the stabilization of water droplets on the solution surface plays an essential role.15 Amphiphilic polymers generally stabilize water droplets and are helpful for the formation of regular pores.14,16 We hypothesize that incorporation of hydrophilic component into hydrophobic PLLA might improve the formation of honeycomb pores. To elucidate this question, nHA will be employed as hydrophilic component with various concentrations in PLLA polymer. More importantly, hydroxyapatite with high similarity to natural bone composition has been demonstrated to enhance cell attachment, proliferation, and differentiation.17–19 Consequently, this work focuses on the development of nHA incorporated honeycomb PLLA films for potential application in bone regeneration. First, the role of hydrophilic nHA in regulating the formation of honeycomb pores in hydrophobic PLLA films will be studied. Second, the effect of nHA in honeycomb-patterned PLLA films on MC3T3-E1 cell functions will be investigated extensively.
Experimental section
Materials
(3s)-cis-3,6-Dimethyl-1,4-dioxane-2,5-dione purchased from Sigma-Aldrich was purified through recrystallization in ethyl acetate. Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) and ammonium phosphate dibasic ((NH4)2HPO4) were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Other solvents and chemicals from commercial suppliers were used as received.Synthesis of nHA
nHA was prepared with a hydrothermal method. In Brief, Ca(NO3)2·4H2O (23.62 g, 100 mmol) and (NH4)2HPO4 (7.89 g, 60 mmol) were completely dissolved in distilled water (1000 mL), respectively. After the pH values of both solution were adjusted at 9–10, Ca(NO3)2·4H2O aqueous solution was added dropwise to (NH4)2HPO4 aqueous solution at an addition speed of 2 mL min−1 under vigorous magnetic stirring when the pH value was maintained at 9–10. After addition, the aging of the precursor solution was performed for 2 days prior to further reaction with DMF as dispersant at 90 °C for another 3 h. Then the solution was cooled down to room temperature, washed with distilled water 4 times, and rinsed with ethanol 3 times. The solvents were removed by centrifugation and the as-prepared nHA was dried at 37 °C as white solid powder. F-HA (15 mm, diameter) films were fabricated by compressing at high pressure.Fabrication of honeycomb films
PLLA (Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, Mw = 20
400 g mol−1, Mw = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 g mol−1) synthesized with methanol as initiator via ring-opening reaction (ROP) of L-lactide monomer was thoroughly dissolved in THF at a concentration of 0.1 g mL−1. Then nHA powders were added to the PLLA solution at a certain concentration with ultrasound for 30 min to obtain homogenous suspension of nHA in solution. The honeycomb films were fabricated via breath-figure method at a relative humidity of 80% and an airflow rate of 50 mL min−1 according to previous reports.6,7 The flat PLLA film as control was prepared with the same procedure, but without humidity and airflow rate.
880 g mol−1) synthesized with methanol as initiator via ring-opening reaction (ROP) of L-lactide monomer was thoroughly dissolved in THF at a concentration of 0.1 g mL−1. Then nHA powders were added to the PLLA solution at a certain concentration with ultrasound for 30 min to obtain homogenous suspension of nHA in solution. The honeycomb films were fabricated via breath-figure method at a relative humidity of 80% and an airflow rate of 50 mL min−1 according to previous reports.6,7 The flat PLLA film as control was prepared with the same procedure, but without humidity and airflow rate.
Characterizations
Transmission electron microscopy (TEM, JEM-2100F, Japan) was employed to observe the morphology of nHA at an acceleration voltage of 200 kV. The phase composition of films was analyzed by wide angle X-ray diffraction (WAXRD, Rigaku Multiflex) with Cu Kα radiation at 40 kV and 100 mA. The film surface and cell morphology were observed using scanning electron microscopy (SEM, JEOL-6360, Japan) at an accelerating voltage of 5 kV.In vitro degradation
To evaluate the degradation of HA in both H-PLLA/nHA and F-HA films, every 10 mg of sample was placed in a α-Minimum Essential Media (α-MEM, Gibco) used for culturing MC3T3-E1 cells and the solution was kept in a shaking water bath at 37 °C for a certain time period. Afterwards, the ionic concentrations of Ca and P in solutions were measured with inductively coupled plasma atomic emission spectroscopy (ICP-AES, IRIS 1000, Thermo Elemental, USA) at 6 h, 12 h, 24 h, 72 h, 120 h, 168 h, and 336 h.Water contact angle and protein adsorption
Prior to the measurements, the films were thoroughly dried under reduced pressure overnight at 37 °C. Water contact angles were determined on XG-7501B (Xuanyichuangxi Industrial Equipment, China). Briefly, 20 μL of distilled water was dropped onto the film and the contact angles were measured after droplets were stable.Both serum medium used for MC3T3-E1 cell culture and fibronectin (FN)/phosphate buffered saline (PBS) (10 μg mL−1) were employed to investigate protein adsorption. Films with known area were immersed in serum medium or FN solution for 4 h in a cell incubator. Subsequently the samples were rinsed with PBS for 6 times and then immersed in 300 μL of 1% sodium dodecyl sulfate (SDS) solution for 0.5 h (four times) to collect proteins on films. The concentrations of proteins in solution were measured with an ELISA plate reader (ELx 800, BIO-TEK) and a MicroBCA protein assay kit (Pierce, Rockford, IL, USA).
Cell culture
The MC3T3-E1 mouse newborn calvaria preosteoblasts (subclone 4, ATCC CRL-2593) were cultured according to the protocol previously reported.6,7,20–23 F-PLLA, H-PLLA, H-PLLA/nHA, and F-HA films (15 mm, diameter) were rinsed in 70% alcohol solution twice, sterilized by 70% alcohol solution for 3 × 30 min, and dried in vacuum at 37 °C overnight. Cells at a density of ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 were seeded on all films in 24-well plates with α-Minimum Essential Media (α-MEM, Gibco) supplemented with 10% fetal bovine serum (Sijiqing, Hangzhou, China) plus 100 U mL−1 penicillin and 100 μg mL−1 streptomycin sulfate in an incubator at 37 °C with humidity of 95% and 5% CO2.
000 cells per cm2 were seeded on all films in 24-well plates with α-Minimum Essential Media (α-MEM, Gibco) supplemented with 10% fetal bovine serum (Sijiqing, Hangzhou, China) plus 100 U mL−1 penicillin and 100 μg mL−1 streptomycin sulfate in an incubator at 37 °C with humidity of 95% and 5% CO2.
MTT assay
To evaluate cell attachment and proliferation, cell numbers were quantified with an MTT assay. At each time point, the cell culture medium was removed and the culture tissue plates were rinsed with PBS 3 times. Then 10 μL of MTT (MajorBiochem, Shanghai, China) solution mixed with 100 μL culture medium was added to each well and the plate was incubated in cell culture incubator for 4 h. Afterwards, 100 μL of SDS–HCl solution was added to each well and mixed thoroughly using the pipette before the incubation for another 4 h. The solution in each well was mixed completely with a pipette again and 100 μL of each solution was transferred to a 96-well plate before the ELISA plate reader (ELx 800, BIO-TEK) was employed to record absorbance at 570 nm. The cell number-optical absorbance standard curve obtained from the absorbance data for known numbers of cells seeded in 24-well plate for 4 h was used to calculate the cell number in each well.Immunofluorescence microscopy and cell morphology
At time points, the MC3T3-E1 cells were fixed with 4% paraformaldehyde (PFA) solution for ∼20 min at room temperature. After PFA was removed, cells were rinsed with PBS 3 times and permeabilized with 0.2% (v/v) Triton X-100. Then cells were rinsed with PBS 3 times and stained with rhodamine-phalloidin (RP) for 2 h in cell incubator. After stained with 4,6-diamidino-2-phenylindole (DAPI) at room temperature, the cytoskeleton and nuclei were observed with confocal laser scanning microscopy (LEICA TCS SD2, Germany). The average cell area was measured from 100 non-overlapping cells at day 1 using the ImageJ software (National Institutes of Health, Bethesda, MD). Focal adhesions (FAs) were stained first with mouse monoclonal anti-vinculin antibody (V 9264, Sigma-Aldrich) for 1 h at 37 °C and rinsed with PBS 5 times. Then, the FAs were further labelled by anti-mouse IgG-FITC antibody (F 0257, Sigma) in cell incubator overnight. The FAs were observed also with confocal laser scanning microscopy. To take deep insight into cell spreading and binding on films at day 1, the fixed cells were dehydrated through gradient ethanol solutions (50%, 70%, 95%, and 100%) for 3 × 10 min at each concentration and finally dried in vacuum overnight. Prior to SEM observation, cells were sputter-coated with a gold–palladium layer.ALP activity and calcification
At day 14, cells growing on films were transferred to centrifuge tubes, rinsed with PBS 3 times, trypsinized, and filled with 5 mL of α-MEM media. The cells were collected and washed with PBS via centrifugation at 1000 rpm for 3 min. The cell pellet was suspended in 1 mL of 0.2% Nonidet P-40 solution and sonicated in ice-water bath for 2 min. The ALP activity and calcium content were determined with a fluorescence-based ALP detection kit (Sigma, St. Louis, MO) and a QuantiChrom calcium assay kit (BioAssay Systems, Hayward, CA), respectively. The calcium deposition on the films was also measured by immersing them in alizarin red S solution (Ricca Chemical, Arlington, TX) for 30 min and photographed with digital camera after the films were thoroughly rinsed with deionized water for 3 × 30 min. Deeper red color indicated better calcium deposition.Gene expression
Gene expression partly referred to previous reports.6,7 After the MC3T3-E1 cells were cultured for 14 days, the cellular RNA was obtained using the mRNA purification kit (Shanghai Shenergy Bioscience & Technology Company, China) and the cDNA was synthesized using the reverse transcriptase MMLV (Promega, USA) and oligo(dT)18 (Takara, Japan) according to the manual. Expressions of alkaline phosphatase (ALP), osteocalcin (OCN), osteopontin (OPN), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) utilized for normalization of any differences in amount of total RNA, were quantified with real-time polymerase chain reaction (PCR) with a Power SYBR® Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) on a thermal cycler with a fluorescence detection system (PTC-200, MJ Research, Watham, MA). The oligonucleotide primers utilized for real-time PCR were: ALP sense, 5′-GCC CTC TCC AAG ACA TAT A-3′; ALP anti-sense, 5′-CCA TGA TCA CGT CGA TAT CC-3′; OCN sense, 5′-CAA GTC CCA CAC AGC AGC TT-3′; OCN anti-sense, 5′-AAA GCC GAG CTG CCA GAG TT-3′; OPN sense, 5′-ACA CTT TCA CTC CAA TCG TCC-3′; OPN anti-sense, 5′-TGC CCT TTC CGT TGT TGT CC-3′; GAPDH sense, 5′-ACT TTG TCA AGC TCA TTT CC-3′; GAPDH anti-sense, 5′-TGC AGC GAA CTT TAT TGA TG-3′.Statistical analysis
Cell studies and ICP-AES measurements were performed in quadruplicates for each group at each time point. For water contact angle and protein adsorption, the tests were performed in triplicates for each group. The statistically significant difference (p < 0.05 or 0.01) between groups was analyzed with the student's t-test.Results and discussion
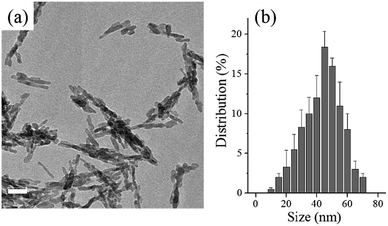
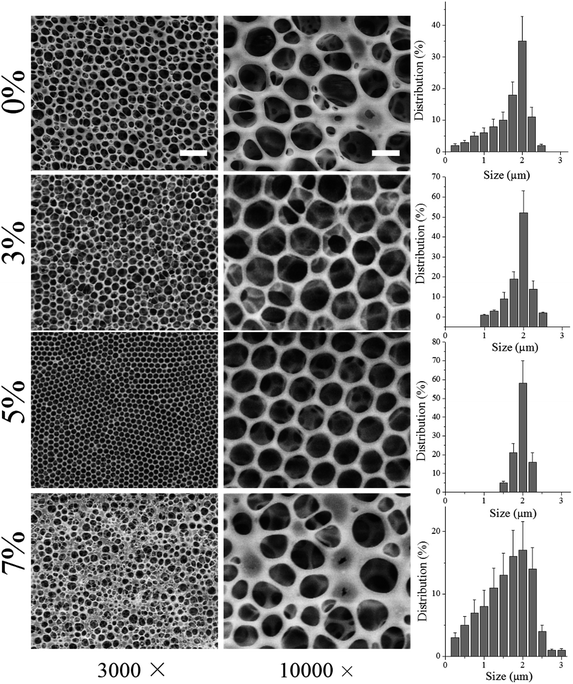
First, nHA particles were synthesized through a hydrothermal method. As demonstrated by TEM image in Fig. 1a and size distribution in Fig. 1b, the as-synthesized nHA particles displayed regular size ranging from 30 to 55 nm and good dispersion. Further, those nHA particles were mixed with PLLA polymer in organic solvent, THF, to fabricate honeycomb films. As shown in Fig. 2, various contents of nHA in PLLA (0 wt%, 3 wt%, 5 wt%, and 7 wt%) were used to evaluate the effect of nHA on the formation of honeycomb pores via breath-figure method. Clearly, the content of nHA exerted a significant influence on the formation of pores. When the content of nHA increased from 0 wt% to 5 wt%, the honeycomb pores had more regular pattern, whereas the highest content of nHA (7 wt%) lowered the quality of honeycomb pattern. The corresponding size distributions in Fig. 2 also demonstrated that the size distribution was narrower on 5 wt% films than on others.The mechanism of breath-figure method consists of three stages in the formation of honeycomb patterns.16,24 First, the water vapor surrounding the polymer solution is condensed into water droplets on the surface of polymer solution due to the sharp drop of surface temperature induced by the fast evaporation of volatile organic solvents, such as CHCl3, CH2Cl2, CS2, toluene, and THF. Second, the water droplets on the solution surface can be stabilized and arranged in a regular array. Eventually, the honeycomb pores are formed once the water droplets, as templates, evaporate completely. In the second stage, the stabilization of condensed water droplets on the solution surface is of importance for the formation of regular honeycomb patterns. As reported earlier, amphiphilic molecules generally assist the stabilization of water droplets and formed more regular pores than those hydrophobic polymers, such as PCL and PLLA.7,14 In a good agreement with previous studies, the hydrophobic homopolymer PLLA could form honeycomb pores, however, with poor regularity as shown in Fig. 2. More interestingly, the incorporation of nHA into PLLA could improve the pore formation and the PLLA solution with 5 wt% nHA formed highly regular pores. Note that inorganic nHA particles were insoluble in THF solvent. Therefore, the nHA particles were stable in PLLA/THF solution. We hypothesize that the dispersed nHA particles in the PLLA/THF solution played a role in stabilizing the water droplets in breath-figure method. Before fabrication of honeycomb films, the nHA particles were completely dispersed in PLLA/THF solution by ultrasound for 30 min and the high viscosity of PLLA/THF solution also maintained the dispersion of nHA particles. However, when the content of nHA reached 7 wt%, nHA precipitation was observed even through ultrasound was applied. The excessive nHA precipitation might disturb the stabilization of water droplets and induce high coalescence of water droplets, causing irregularity of pore size on 7 wt% honeycomb films. The honeycomb structure can also be mediated by organic solvents used for fabrication. When the solvent is denser than water, the water droplet could not penetrate solvent and single-layer pores are formed. On the contrary, denser than the organic solvent, water droplets can penetrate the solvent to form multi-layer pores. In this study, multi-layer porous structure was observed in all films (0 wt%, 3 wt%, 5 wt%, and 7 wt%) due to the lower density of THF than water.
In order to perform cell study to investigate the effect of nHA in honeycomb PLLA films on MC3T3-E1 cell functions. Honeycomb PLLA films with 5 wt% nHA (abbreviated as H-PLLA/nHA) was selected as samples for in vitro study and flat PLLA films, honeycomb PLLA films with 0 wt% nHA, and flat HA films (abbreviated as F-PLLA, H-PLLA, and F-HA, respectively) were used as controls.
To verify the chemical composition of F-PLLA, H-PLLA, H-PLLA/nHA, and F-HA films, WAXRD test was performed as shown in Fig. 3a. Reflection planes (110)/(200), and (203) at 19° and 22° corresponding to the α-form crystal structure, the most common polymorph for solution cast and melt processed PLLA films with an orthorhombic unit cell, of semi-crystalline PLLA were observed.25,26 Apparently, F-PLLA and H-PLLA showed the same XRD spectrum, indicating the identical crystals. The main indices of (002), (102), (210), (211), (300), (202), and (310) corresponding to HA crystals at 30°, 32°, 33°, 37°, 39°, 40°, and 46° were found in the WAXRD spectrum of F-HA films, which was consistent with other reports.19,27–29 When the nHA was incorporated into PLLA films, the reflection planes assigned to both PLLA and HA appeared in H-PLLA/nHA films.
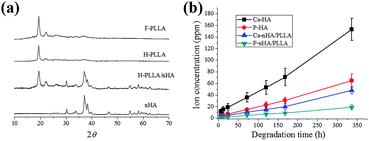 | ||
| Fig. 3 (a) XRD spectra of F-PLLA, H-PLLA, H-PLLA/nHA, and F-HA films. (b) Ca and P ion concentrations of H-PLLA/nHA and F-HA films in α-MEM media at different degradation time points. | ||
As mentioned in the introduction section, the incorporation of HA into PLLA might perform functions in regulating MC3T3-E1 cell behavior. Herein, the in vitro degradation of HA in H-PLLA/nHA and F-HA was studied prior to cell study using ICP-AES to measure the Ca and P ionic concentrations as indicated in Fig. 3b. HA in both H-PLLA/nHA and F-HA films could degrade gradually to generate Ca and P ions in cell culture medium. Clearly, the F-HA films had much faster Ca and P release compared with H-PLLA/nHA because of the smaller HA content exposed to culture medium in H-PLLA/nHA films than F-HA films. This data indicated that nHA had certain degradation capability, which was consistent with earlier findings that nHA had higher dissolution rate and was more resorbable than conventional HA.
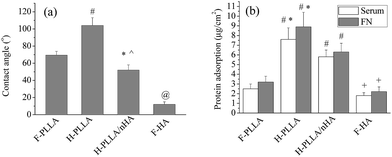
The surface topography and chemistry can influence the wettability.7,30–32 In a good agreement with previous findings that honeycomb pores were demonstrated to be more hydrophobic by producing air pockets between water droplets and substrate surface when the water droplets were much larger than the pore size, the water contact angle increased from 69 ± 7° on F-PLLA films to 103 ± 12° on H-PLLA films. More interestingly, the H-PLLA/nHA films, even though having similar topography to that of H-PLLA films, presented lower water contact angle of 45 ± 8° than H-PLLA films, which should be attributed to the incorporation of nHA particles. HA is known to be hydrophilic materials with a water contact angle of ∼10°,33 as also demonstrated in Fig. 4a. The nHA particles on the surface of H-PLLA/nHA films facilitated the spreading of water droplets to induce lower contact angles. Note that the H-PLLA/nHA films were fabricated via breath-figure method with condensed water droplets as templates. Another reason that might contribute to the improved wettability of H-PLLA/nHA is the pore wall covered with a nHA layer. During fabrication, the nHA might stabilize condensed water droplets by surrounding them with nHA particles, which formed pore walls consisted of nHA particles. Therefore, once the water droplets used for the contact angle test were dropped onto the H-PLLA/nHA films, they might easily penetrate into pores, causing lower contact angles instead of improving the formation of air pockets to induce higher hydrophobicity.
The honeycomb pores and nHA particles in H-PLLA/nHA films also exerted a significant impact on protein adsorption, as demonstrated in Fig. 4b. Both serum and FN protein adsorptions were enhanced on H-PLLA films compared with F-PLLA films, due to the enlarged surface area by porous structure as demonstrated previously.7 Interestingly, both adsorptions were suppressed on H-PLLA/nHA films compared with H-PLLA films, which might be attributed to the availability of nHA particles on H-PLLA/nHA films. As reported earlier that hydrophobic surface favored protein adsorption,34 whereas hydrophilic surface lowers protein adsorption, hydrophilic HA materials, as confirmed in Fig. 4b, showed significantly lower serum and FN adsorptions compared with other films. The availability of nHA particles on H-PLLA/nHA films, especially in pore walls, was also expected to suppress the serum and FN adsorptions compared with H-PLLA films.
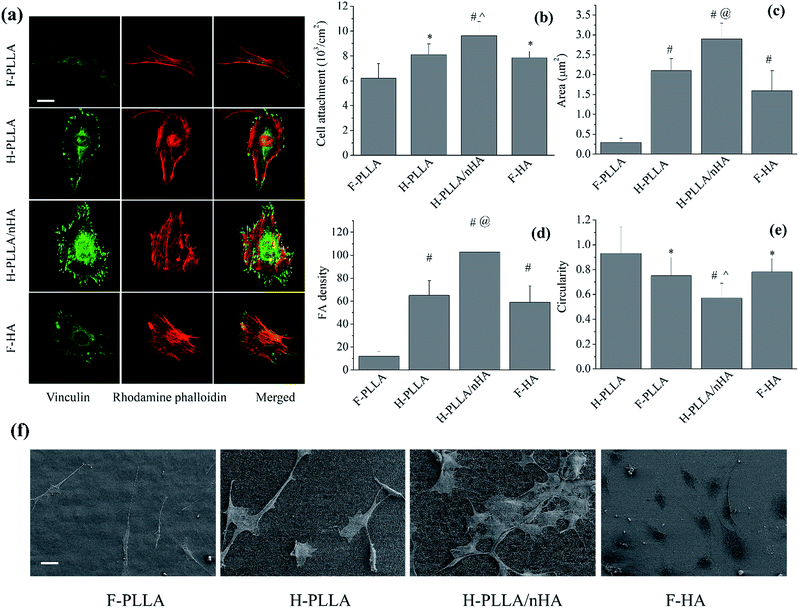
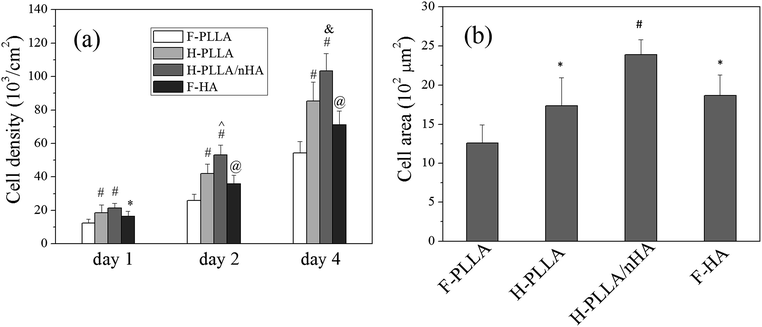
Cell attachment and adhesion were significantly influenced by honeycomb pores and HA as well (Fig. 5). Vinculin-stained images (Fig. 5a) clearly showed that cells on H-PLLA/nHA films had the highest density of FAs among all films, whereas those on F-PLLA films had fewer visible FAs compared with others. The combined RP-stained and vinculin-stained images demonstrated that FAs on honeycomb films, especially H-PLLA/nHA films, not only were distributed along the periphery of cytoskeleton, as observed on F-PLLA and F-HA films, but also spread over the cell body, which agreed with previous findings.6,7 The FAs were also quantified by ImageJ software in terms of FA area, FA density defined as average number of FAs per cell, and circularity, defined as 4π × area/perimeter2 (Fig. 5c–e). Consistent with Fig. 5a, FA area and FA density on H-PLLA/nHA films were markedly enhanced compared with others, whereas both parameters showed the lowest values on F-PLLA films. The circularities of FAs were measured to be ∼0.92, ∼0.72, ∼0.50, and ∼0.74 on F-PLLA, H-PLLA, H-PLLA/nHA, and F-HA films, respectively. The circularity data indicated that FAs on H-PLLA, H-PLLA/nHA, and F-HA films, especially H-PLLA/nHA films, were significantly aligned. These analyses of FAs, together with vinculin-stained images, strongly demonstrated that cell adhesion was enhanced on H-PLLA/nHA films compared with other groups. The cell attachment, represented by cell number determined by MTT assay, was also studied as shown in Fig. 5b. Consistently, cells showed the highest density on H-PLLA/nHA films, while those had the lowest density on F-PLLA films. Further, cell morphology was investigated using SEM observation (Fig. 5f). Cells on F-PLLA films had relatively smaller spreading area compared with those on other films, especially H-PLLA/nHA, on which cells spread and flattened much better. Taking deeper insight into the cell body, we found that cells on H-PLLA/nHA films had more filopodia, indicating better adhesion. On the other hand, the SEM images also prominently demonstrated that H-PLLA/nHA films supported the highest cell density and the F-PLLA films supported the lowest cell density, which partly indicated that cell proliferation was promoted on H-PLLA/nHA films.
In our present study, the factors contributing to cell behavior can be exemplified as surface topographical features, chemistry, and mechanical properties. The porous structure in H-PLLA films effectively increased the surface contact area with proteins, resulting in enhanced both serum and FN protein adsorptions. The integrins play an important role in bridging cells and ECM proteins, such as FN, COL I, and vitronectin by binding ECM proteins with the external end and the cytoskeleton with adapter proteins including talin, filamin, vinculin, and α-actinin, which form the integrin-adapter protein–cytoskeleton complex, namely FAs.35,36 Thus, the adsorption of ECM proteins on films before the cell contact with films is of importance for cell adhesion. However, the cells did not spread into the pores because of the much smaller pore size than cell dimension, meaning that the adsorbed protein in the pores might not contribute much to the formation of FAs, even though the adsorbed ECM proteins from culture media might be gradually released to provide ECM proteins. Earlier reports suggested that the protein concentration on the flat-top area of the honeycomb films was actually lower than on flat films and concluded that the better cell adhesion could not only be attributed to the protein adsorption, but also topographical features.7 Due to the multi-layer porous architecture, the actual surface area, the real protein concentration on the flat-tops, and the protein concentration at unit area could not be calculated here. The honeycomb pores, a special microenvironment, might influence the cell response to films. It was suggested, previously, that the fibril-like aggregates of FN locating on the periphery of the pores might determine the formation of FAs.4,37–40 However, it is still unclear if this mechanism worked in our present study. The H-PLLA/nHA films, with lower protein adsorption than H-PLLA films, nevertheless, showed better cell adhesion than H-PLLA films. This can only be attributed to the effect of nHA particles. Previously, a sol–gel technique was applied to modify titanium surface with HA coating and the data showed enhanced osteoblast attachment on the HA-coated surface compared with the uncoated one.17,18 In another study more similar to our study, nano-featured HA coating and micrometer-scale HA coating on titanium were employed to investigate the effect of nanocrystalline HA coatings on the activity of osteoblasts.41 The results clearly showed that the HA coatings, especially nanofeatured HA coating, strongly promoted cell adhesion, proliferation, and osteoblastic gene and protein expressions. It was suggested that nHA coating might promote the interactions of selected serum protein(s), which in turn could enhance osteoblast adhesion. Compared with H-PLLA/nHA films, the cell adhesion on F-HA was suppressed, but still comparable to H-PLLA films. As an inorganic material, HA has a similar chemical composition to the mineral phase of human bone, benefiting the osteoblast functions and bone modeling.18 Hence, the F-HA films stimulated better cell adhesion than F-PLLA films. However, deep insight into the surface topographies of H-PLLA and H-PLLA/nHA films clearly indicated that H-PLLA/nHA films had more uniform shape of honeycomb arrays than H-PLLA ones. Therefore, to rule out the effect of the shape of honeycomb arrays on cell functions, we, meanwhile, performed cell study on those H-PLLA/nHA films after removal of nHA on the surface exposed to solution (abbreviated as H-PLLA/nHA-R) together with F-PLLA, H-PLLA, H-PLLA/nHA, and F-HA films. The H-PLLA/nHA-R films were prepared by immersing them in PBS solution until no ions were released, which was monitored by ICP-AES test. The comparison between H-PLLA and H-PLLA/nHA-R films in terms of cell adhesion, attachment, proliferation, and differentiation was systematically investigated. However, no difference was found as shown in Fig. S1–S3.† Consequently, the shape of honeycomb arrays did not influence cell functions in our study and the cell study on H-PLLA/nHA-R was not mentioned in the following sections to avoid redundant description.
Better cell adhesion also induced better cell proliferation as demonstrated in Fig. 6. The quantification of cell density at time points using MTT assay (Fig. 6a) indicated that cell proliferation was faster on H-PLLA films, H-PLLA/nHA films, and F-HA films, especially on H-PLLA/nHA films, than on F-PLLA films. The cell spreading area at day 1 was measured from fluorescent images using ImageJ software as shown in Fig. 6b. In agreement with Fig. 5f, cells had the significantly larger area on H-PLLA/nHA films and the smallest area on F-PLLA films.
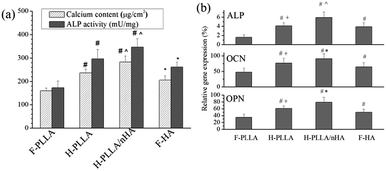
Moreover, the cell differentiation was also influenced by honeycomb pores and HA, as demonstrated by calcification and ALP activity, two indicators of osteogenesis, in Fig. 7a. Both parameters were promoted significantly on H-PLLA, H-PLLA/nHA, and F-HA films compared with F-PLLA films and H-PLLA/nHA films induced the best values. The mRNA expression of bone-specific differentiation markers, ALP, OCN, and OPN, were also analyzed with real-time PCR as shown in Fig. 7b. The expression levels of those markers indicated the same trend as that observed in Fig. 7a, which demonstrated further that honeycomb pores enhanced cell differentiation, especially when incorporated with nHA particles.
Due to the faster proliferation, cells on H-PLLA/nHA films reached confluence earlier than other films and the cell differentiation was also launched earlier on H-PLLA/nHA films. This should contribute to the better differentiation on H-PLLA/nHA films. However, the Ca and P ions might also be involved in the differentiation process. The mitogen-activated protein kinase (MAPK) signaling pathways have been involved in cell proliferation, differentiation, and other cellular responses induced by extracellular stimuli.42 As one of the four subgroups in MAPK family, Extracellular signal-regulated kinases (ERKs) have been found to be critical to osteoblast adhesion, spreading, migration, integrin expression, and differentiation.42 Cellular Ca ion has been demonstrated to be crucial to ERK 1/2 activation in osteoblasts.43 Therefore, the Ca ion released from nHA particles might be responsible for good cell functions on H-PLLA/nHA films. A previous study suggested that the extracellular Ca ion influenced ALP activity and mineralization of MC3T3-E1 cells.44 Also, extracellular Ca ions have stimulatory effect on ALP activity through transcription factors, such as SMAD3 and TGF family.45 The Ca ions on the hydroxycarbonate (HCA) surface up-regulate the expression of TGF-1 in ROS17/2.8 osteoblastic cells.46 Besides, P ions resulted from the activity of the tissue non-specific alkaline phosphatase (TNAP) and bone remodeling in the bone matrix is believed to function as a specific signal for skeletal cells, which influences different genes expression and cell functions, such as proliferation, differentiation, mineralization, and apoptosis.47 Consequently, the Ca and P ions released from nHA or HA might enhance cell adhesion, proliferation, and differentiation.
Conclusion
In our present study, we fabricated honeycomb PLLA films with various nHA compositions to evaluate the effect of nHA on self-assembly of honeycomb pores in PLLA films, finding that 5 wt% nHA in PLLA solution resulted in the most regular honeycomb pores. F-PLLA, H-PLLA, H-PLLA/nHA, and F-HA films were selected to investigate water contact angle test, protein adsorptions including FN and serum, and MC3T3-E1 cell responses. Honeycomb pores on H-PLLA films enhanced hydrophobicity compared with F-PLLA films, whereas the pores and nHA particles on H-PLLA/nHA lowered the hydrophobicity compared with H-PLLA. Both the FN and serum protein adsorptions were increased on H-PLLA films compared with F-PLLA, but decreased significantly on H-PLLA/nHA films compared with H-PLLA films. The MC3T3-E1 cell adhesion, spreading, proliferation, and differentiation were enhanced on honeycomb films, especially H-PLLA/nHA films, compared with F-PLLA films and they did not show difference between H-PLLA films and F-HA films. Our present study provides one promising modality to fabricate engineered materials with honeycomb pores incorporating nHA particles for bone repair.Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (31271031), the International Cooperation Project of the Ministry of Science and Technology of China (2013DFB50280), the National High Technology Research & Development Program of China (863 Program) (no. 2014AA021202), the Key Medical Program of Science and Technology Development of Shanghai (no. 12441902802), and the Major Program of Natural Science Foundation of Shanghai, China (11JC1416302).Notes and references
- J. Y. Wong, J. B. Leach and X. Q. Brown, Surf. Sci., 2004, 570, 119 CrossRef CAS PubMed.
- D. E. Discher, P. Janmey and Y. L. Wang, Science, 2005, 310, 1139 CrossRef CAS PubMed.
- L. M. Y. Yu, N. D. Leipzig and M. S. Shoichet, Mater. Today, 2008, 11, 36 CrossRef CAS.
- J. R. McMillan, M. Akiyama, M. Tanaka, S. Yamamoto, M. Goto, R. Abe, D. Sawamura, M. Shimomura and H. Shimizu, Tissue Eng., 2007, 13, 789 CrossRef CAS.
- B. Wang, Z. W. Mao, X. A. Meng, W. J. Tong and C. Y. Gao, Colloids Surf., B, 2010, 76, 38 CrossRef CAS PubMed.
- X. Wu and S. Wang, Polymer, 2014, 55, 1756 CrossRef CAS PubMed.
- X. Wu and S. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4966 CAS.
- A. Tsuruma, M. Tanaka, S. Yamamoto and M. Shimomura, Colloids Surf., A, 2008, 313, 536 CrossRef PubMed.
- Y. Fukuhira, H. Kaneko, M. Yamaga, M. Tanaka, S. Yamamoto and M. Shimomura, Colloids Surf., A, 2008, 313, 520 CrossRef PubMed.
- Y. Yang, N. Bajaj, P. Xu, K. Ohn, M. D. Tsifansky and Y. Yeo, Biomaterials, 2009, 30, 1947 CrossRef CAS PubMed.
- N. Nasongkla, X. Shuai, H. Ai, B. D. Weinberg, J. Pink, D. A. Boothman and J. M. Gao, Angew. Chem., Int. Ed., 2004, 43, 6323 CrossRef CAS PubMed.
- G. S. Kwon and K. Kataoka, Adv. Drug Delivery Rev., 1995, 16, 295 CrossRef CAS.
- X. Jiang, T. Zhang, L. Xu, C. Wang, X. Zhou and N. Gu, Langmuir, 2011, 27, 5410 CrossRef CAS PubMed.
- B. H. Zhao, J. Zhang, X. D. Wang and C. X. Li, J. Mater. Chem., 2006, 16, 509 RSC.
- P. Escalé, L. Rubatat, L. Billon and M. Save, Eur. Polym. J., 2012, 48, 1001 CrossRef PubMed.
- U. H. F. Bunz, Adv. Mater., 2006, 18, 973 CrossRef CAS.
- J. Harle, H. W. Kim, N. Mordan, J. C. Knowles and V. Salih, Acta Biomater., 2006, 2, 547 CrossRef PubMed.
- M. Sato, E. B. Slamovich and T. J. Webster, Biomaterials, 2005, 26, 1349 CrossRef CAS PubMed.
- W. Chen, T. Long, Y. J. Guo, Z. A. Zhu and Y. P. Guo, RSC Adv., 2014, 4, 185 RSC.
- X. Wu and S. Wang, Adv. Healthcare Mater., 2013, 2, 326 CrossRef CAS PubMed.
- J. Wei, X. Wu, C. Liu, J. Jia, S. J. Heo, S. E. Kim, Y. T. Hyun and J. W. Shin, J. Am. Ceram. Soc., 2009, 92, 1017 CrossRef CAS PubMed.
- H. Zhou, X. Wu, J. Wei, X. Lu, S. Zhang, J. Shi and C. Liu, J. Mater. Sci.: Mater. Med., 2011, 22, 731 CrossRef CAS PubMed.
- H. Zhou, J. Wei, X. Wu, J. Shi, C. Liu, J. Jia, C. Dai and Q. Gan, J. Mater. Sci.: Mater. Med., 2010, 21, 2175 CrossRef CAS PubMed.
- M. Hernandez-Guerrero and M. H. Stenzel, Polym. Chem., 2012, 3, 563 RSC.
- J. Kobayashi, T. Asahi, M. Ichiki, A. Oikawa, H. Suzuki, T. Watanabe, E. Fukada and Y. Shikinami, J. Appl. Phys., 1995, 77, 2957 CrossRef CAS PubMed.
- C. Marega, A. Marigo, V. Dinoto, R. Zannetti, A. Martorana and G. Paganetto, Makromol. Chem., 1992, 193, 1599 CrossRef CAS.
- X. Wu, J. Wei, X. Lu, Y. Lv, F. Chen, Y. Zhang and C. Liu, Biomed. Mater., 2010, 5, 035006 CrossRef PubMed.
- F. Mohandes and M. Salavati-Niasari, RSC Adv., 2014, 4, 25993 RSC.
- K. L. Lin, Y. L. Zhou, Y. Zhou, H. Y. Qu, F. Chen, Y. J. Zhu and J. Chang, J. Mater. Chem., 2011, 21, 16558 RSC.
- Q. Yu, X. Li, Y. X. Zhang, L. Yuan, T. L. Zhao and H. Chen, RSC Adv., 2011, 1, 262 RSC.
- R. V. Goreham, A. Mierczynsk, L. E. Smith, R. Sedev and K. Vasilev, RSC Adv., 2013, 3, 10309 RSC.
- E. Min, K. H. Wong and M. H. Stenzel, Adv. Mater., 2008, 20, 3550 CrossRef CAS.
- D. Aronov, R. Rosen, E. Z. Ron and G. Rosenman, Process Biochem., 2006, 41, 2367 CrossRef CAS PubMed.
- R. D. K. Misra, W. W. Thein-Han, T. C. Pesacreta, K. H. Hasenstein, M. C. Somani and L. P. Karjalainen, Acta Biomater., 2009, 5, 1455 CrossRef CAS PubMed.
- M. C. Siebers, P. J. ter Brugge, X. F. Walboomers and J. A. Jansen, Biomaterials, 2005, 26, 137 CrossRef CAS PubMed.
- K. Anselme, Biomaterials, 2000, 21, 667 CrossRef CAS.
- S. Yamamoto, M. Tanaka, H. Sunami, K. Arai, A. Takayama, S. Yamashita, Y. Morita and M. Shimomura, Surf. Sci., 2006, 600, 3785 CrossRef CAS PubMed.
- H. Sunami, E. Ito, M. Tanaka, S. Yamamoto and M. Shimomura, Colloids Surf., A, 2006, 284, 548 CrossRef PubMed.
- M. Tanaka, A. Takayama, E. Ito, H. Sunami, S. Yamamoto and M. Shimomura, J. Nanosci. Nanotechnol., 2007, 7, 763 CrossRef CAS PubMed.
- S. Yamamoto, M. Tanaka, H. Sunami, E. Ito, S. Yamashita, Y. Morita and M. Shimomura, Langmuir, 2007, 23, 8114 CrossRef CAS PubMed.
- Y. Tang, B. Wen, Y. Wu, T. Yu, W. Wang and Y. Wang, Cent. Eur. J. Biol., 2012, 7, 54 CrossRef CAS PubMed.
- H. J. Zhou, J. Wei, X. H. Wu, J. L. Shi, C. S. Liu, J. F. Jia, C. L. Dai and Q. Gan, J. Mater. Sci.: Mater. Med., 2010, 21, 2175 CrossRef CAS PubMed.
- S. Choudhary, S. Wadhwa, L. G. Raisz, C. Alander and C. C. Pilbeam, J. Bone Miner. Res., 2003, 18, 1813 CrossRef CAS PubMed.
- A. Lazary, B. Balla, J. P. Kosa, K. Bacsi, Z. Nagy, I. Takacs, P. P. Varga, G. Speer and P. Lakatos, Biomaterials, 2007, 28, 393 CrossRef CAS PubMed.
- H. Sowa, H. Kaji, T. Yamaguchi, T. Sugimoto and K. Chihara, J. Bone Miner. Res., 2002, 17, 1190 CrossRef CAS PubMed.
- H. Matsuoka, H. Akiyama, Y. Okada, H. Ito, C. Shigeno, J. Konishi, T. Kokubo and T. Nakamura, J. Biomed. Mater. Res., 1999, 47, 176 CrossRef CAS.
- S. Khoshniat, A. Bourgine, M. Julien, M. Petit, P. Pilet, T. Rouillon, M. Masson, M. Gatius, P. Weiss, J. Guicheux and L. Beck, Bone, 2011, 48, 894 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Cell adhesion, attachment, proliferation, and differentiation data for comparison between H-PLLA and H-PLLA/nHA-R films. See DOI: 10.1039/c4ra13843k |
| This journal is © The Royal Society of Chemistry 2015 |