Preparation and tribological properties of Ti3C2(OH)2 nanosheets as additives in base oil
Abstract
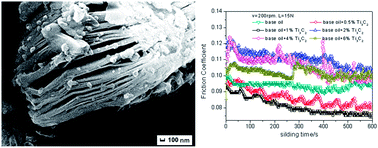
The two-dimensional Ti3C2(OH)2 nanosheets were synthesized by exfoliation of laminated Ti3AlC2 in HF and characterized by X-ray diffractometry, SEM, TEM, and HRTEM. The results indicated that the Ti3C2(OH)2 compounds had 2D layer-like structures with a thickness of 10–20 nm, and the fabrication process of Ti3C2(OH)2 nanosheets was discussed on the basis of the experimental facts. The tribological properties of Ti3C2(OH)2 nanosheets as additives in base oil were investigated using a UMT-2 ball-on-disc tribotester. Under the determinate conditions, the friction coefficient of the base oil containing Ti3C2(OH)2 nanosheets was lower than that of the base oil under 15 N load with 200 rpm, and decreased with increasing mass fraction of Ti3C2(OH)2 nanosheets when it was <1.0 wt%. A combination of sliding friction, and stable tribofilm on the rubbing surface could explain the improved tribological properties of Ti3C2(OH)2 nanosheets as additives.

 Please wait while we load your content...
Please wait while we load your content...