Temperature and CO2 responsive polyethylenimine for highly efficient carbon dioxide release†
Johannes Kainz,
Patrick David Leonidas Werz,
Carsten Troll and
Bernhard Rieger*
WACKER-Lehrstuhl für Makromolekulare Chemie, Technische Universität München, Lichtenbergstraße 4, 85747 Garching b. München, Germany. E-mail: rieger@tum.de; Fax: +49 89 289 13562; Tel: +49 89 289 13571
First published on 24th December 2014
Abstract
Post combustion carbon dioxide capture with aqueous polymer solutions is a field of major interest. Via acylation of commercially available polyethylenimine (b-PEI) with butyric anhydride, lower critical solution temperature (LCST) behaviour together with a reversible pH shift was found in water as well as in CO2 containing aqueous solutions. As expected, a low CO2 absorption capability of the acylated thermosensitive b-PEI was measured in a stirred tank reactor. However, the observed improved CO2 release in the desorption process associated with the LCST behaviour of the polymer is a “green” tool to face high efficiency loss in standard CO2 capturing processes.
Climate change is a major problem for mankind. Since the industrial revolution the combustion of fossil fuels like coal, natural gas and oil have led to an accumulation of carbon dioxide in the atmosphere.1 In 2011, 35 gigatons of CO2 were emitted from fuel burning devices and the cement industry, corresponding to a 54% increase as compared to the level in 1990.1 Carbon dioxide capture and storage (CCS) is considered as one of the options to reduce atmospheric emissions of CO2 from human activities.2,3 The captured CO2 is a renewable C1 feedstock4 and can be utilized, e.g. for the production of green fuels5 or CO2 containing polymers.6–8 Within the CCS technology, the post combustion CO2 capture shows the greatest potential because it can be retrofitted to existing fossil fuelled power plants and other CO2 emitting devices.9 Usually, aqueous solutions of monoethanolamine react with CO2 at 40 °C (ref. 10) through a zwitterion mechanism to form bicarbonates.11 The regeneration of the monoethanolamine at elevated temperatures (100–140 °C),10 is a high energy consuming process and leads to an efficiency penalty.12 Said high energy consumption is the main drawback of this standard amine process, so that sorbents like metal–organic frameworks,13,14 solid porous absorbent materials,14,15 porous polymer networks16 and ionic liquids17 have been studied intensively in order to overcome the aforementioned problem.
Thermo- and CO2-responsive polymers18,19 are studied intensively to improve the regeneration of aqueous sorbents and, therefore, the temperature dependent solubility of a polymer known as lower critical solution temperature (LCST) is addressed. This entropically driven effect can only be observed in polymer systems and may be seen as the most promising tool to reduce the high efficiency loss during the regeneration process which is mainly caused by the high heat capacity of the aqueous amine solutions.20 Below the LCST, the solution is a one phase system and above said specific temperature, the polymer chains collapse and precipitate from solution. In 2012, Bergbreiter et al. reported that the LCST behaviour of polymers with acidic or basic side groups correlates with a pH shift.21 Zhao et al. outlined a reversible thermosensitive behaviour in CO2 containing solutions.22 Recently, Hoshino et al. reported the absorption and desorption behaviour of CO2 in micro- and nanogel particles (GPs) consisting of a copolymer of N-isopropylacrylamide (NIPAm) and N-[3-(diethylamino)propyl]methacrylamide (DMAPM)23 and extended this concept of micro- and nanogel particles to microgel films in a wet environment.24
In 2011, Kim et al. reported that branched polyethylenimine can be equipped with thermosensitive behaviour by simple acylation with several anhydrides.25 Polyethylenimines (PEIs) are commercially available, wherein it is known that the linear form is capable to absorb CO2.26 Several publications27–30 report on PEI as an absorbing material for CO2 immobilized on solid materials.
Herein, we present the improved release of carbon dioxide within the regeneration process in an aqueous solution of thermosensitive acylated polyethylenimine. Our goal is to provide thermosensitive behaviour and the ability of CO2 absorption in a commercially available homopolymer in order to design a polymer based high efficient CO2 capturing process.
Branched polyethylenimine obtained via cationic polymerisation of aziridine is commercially available as Lupasol® and is a well-known polymer with primary, secondary and tertiary amine groups in the polymer backbone.31 b-PEI purchased from Sigma-Aldrich was dissolved in methanol, mixed with triethylamine and was acylated using 0.75 and 1.0 equivalents, respectively, of butyric anhydride according to the procedure of Kim et al.25 1H and 13C-NMR of the purchased polymer samples (Fig. S2 and S3 in the ESI†) are in agreement with recently published data.32 An excerpt of the structure of the acylated polymer is given in Fig. 1.
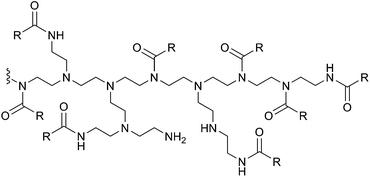 | ||
| Fig. 1 Excerpt of the structure of acylated branched polyethylenimine with butyric anhydride. R = CH2CH2CH3. | ||
The acylation reaction was confirmed by 1H and 13C NMR (Fig. S4 and S5†). Thermosensitive properties especially cloud point and lower critical solution temperature were determined by recording the transmittance 2 wt% polymer solutions of nBu-PEI-0.75 and nBu-PEI-1.0 in a standard UV-Vis spectrometer. Differential scanning calorimetry (DSC) measurements were performed as well to determine Tg. The LCST and DSC data are shown in Table 1.
| Polymer | b-PEI | nBu-PEI-0.75 | nBu-PEI-1.0 |
|---|---|---|---|
| TLCST | — | 19 | 14 |
| Tg | −54 | −9 | −8 |
As reported earlier by Kim et. al., the acylated compounds exhibit a LCST below room temperature (nBu-PEI-0.75 TLCST = 19 °C and nBu-PEI-1.0 TLCST = 14 °C). As expected, the LCST of the b-PEI acylated with 1.0 eq. butyric anhydride shows a lower LCST as compared to the nBu-PEI-0.75 due to the higher degree of acylation. The DSC results indicate a clear shift in the glass transition point after the acylation of the b-PEI. The implementation of unpolar alkyl group via treatment of polyethylenimine with butyric anhydride led to lower mobility of the polymer chains and therefore to a higher glass transition point.
To further address the thermosensitive behaviour, to show the improved carbon dioxide release and the CO2 absorption and desorption capability of nBu-PEI-0.75 and nBu-PEI-1.0, a stirred tank reactor was used. With this unique reactor system, it is possible to get a detailed and complete overview of all relevant parameters (CO2 flow, pressure, temperature, pH) in the polymer solutions during the absorption and desorption of CO2 (Fig. S6†).
The polymer samples (2 wt% polymer solutions of nBu-PEI-0.75 and nBu-PEI-1.0 in Millipore® water) were charged into the reactor and carbon dioxide was added to the solution. UV-Vis measurements after release of the CO2 overpressure were recorded to determine the LCST in CO2 containing aqueous solution (Fig. 2).
The cloud point in carbon dioxide containing water is shifted to higher temperatures compared to the CO2 free system. Furthermore, the observed phase transition is broadened in the presence of CO2.22 As a result, these measurements clearly indicate that it is possible to introduce thermosensitive properties to branched polyethylenimine in a CO2 containing aqueous solution.
The additional pH shift in connection with the LCST effect is of particular relevance for the release of CO2 in the desorption process because of the proton driven decomposition of bicarbonate moieties.21 Due to the fact that the acylated b-PEI has some basic groups within the polymer even after acylation, a connection between the phase transition above the LCST temperature and a shift in the pH value was expected in the present case. In order to demonstrate this correlation, the polymer solution was charged into our reactor setup. The pH value was measured and plotted against the temperature in the solution using a digital pH electrode. To ensure a one phase system, of nBu-PEI-0.75 and nBu-PEI-1.0 solutions were cooled below their LCST to 10 °C. Subsequently, each polymer solution was heated above the LCST to 50 °C and then cooled to 10 °C again. This procedure was repeated five times. A pH shift of more than one unit was observed and obviously correlated with the LCST of the two acylated species (Fig. 3). The pH shift was reversible for five heating and cooling cycles and was almost in the same range for both polymer samples.
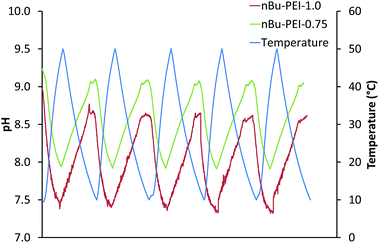 | ||
| Fig. 3 Reversible change in pH of nBu-PEI-0.75 and nBu-PEI-1.0 before CO2 absorption over five heating and cooling cycles. | ||
According to the transmittance curves in Fig. 2, we were able to address a LCST behaviour in water and in CO2 containing solutions. Additionally, the phase transition before the absorption was correlated with a reversible shift in pH over five heating and cooling cycles. However, we had to analyse the CO2 absorbed and desorbed with the thermosensitive b-PEI polymer solution. As described by Theato et al.,18 thermosensitive and CO2 responsive amine containing polymer systems show a low absorption capacity.27 The amount of CO2 was measured by two digital flow meters and recorded online. For the absorption reaction, the CO2 pressure was set to one bar. The pressure was regulated by a digital pressure valve. The temperature was kept constant at 10 °C below the LCST of nBu-PEI-0.75 and nBu-PEI-1.0 during the absorption process and during the release of the overpressure at the beginning of desorption. After the release of the overpressure, the temperature was set to 95 °C and monitored together with the pH value during the absorption and desorption process (Fig. S7†). The amount of CO2 absorbed and desorbed is calculated from the integral of the flow curves. In order to determine the amount of CO2 absorbed or desorbed by the polymer, the CO2 absorbed by water is subtracted from the calculated values (Table 2). 490 g water desorbed 3.36 g CO2 and water vapour due to the high desorption temperature of 95 °C corresponding to 2.71 g absorbed CO2. In order to relate the outlined absorption and desorption capacity of the acylated polymer systems with a standard amine system, the amount of CO2 absorbed and desorbed by a 2 wt% monoethanolamine solution is also given (Table 2).
As expected, rather low amounts of CO2 were absorbed and desorbed by the acylated polymers compared to a 2 wt% monoethanolamine solution due to the high degree of acylation in case of nBu-PEI-0.75 and nBu-PEI-1.0. Reactive primary and secondary amine side groups were blocked and were not available for the absorption reaction. Low polymer concentrations of two weight percent for of nBu-PEI-0.75 and nBu-PEI-1.0 were another reason for the observed values. A comparison of the thermosensitive properties, absorbed and desorbed CO2 and pH values of acylated branched polyethylenimine and poly(N-isoporpylacrylamide(NIPAm)-co-N-[3-(diethylamino)propyl]methacrylamide (DMAPM))23 is given in Table S1.†
To provide evidence for the impact of the proton driven decomposition of HCO3− within the desorption process, the desorption curves and the pH value during this process were considered in detail. To remove non-absorbed CO2 in the gas phase, the overpressure was reduced to atmospheric pressure first. Then the temperature was continuously increased to 95 °C and the CO2 flow was recorded during this continuous increase in temperature together with the pH value. The proof for thermosensitive properties of acylated b-PEI and a correlated decrease in pH during the continuous increase of temperature in the regeneration process is shown in Fig. 4.
 | ||
| Fig. 4 pH trend of nBu-PEI-0.75, nBu-PEI-1.0 and water versus a continuous rise in temperature during the regeneration process. | ||
While the pH value of water increases continuously due to desorption of CO2, the pH values of the polymer solutions decrease during increase in temperature to 90 °C. This pH drop must obviously be caused by the LCST effect of the acylated polymers nBu-PEI-0.75 and nBu-PEI-1.0 in the CO2 containing solutions. The LCST effect is associated with a release of protons and a reduction in pH during the phase transition of the acylated polyethylenimine. Apart from the decrease in pH associated with the LCST effect, a pH rise was observed for both the acylated b-PEIs and water caused by the additional thermal decomposition of bicarbonate at 95 °C.
In addition to the thermally driven desorption of CO2, the LCST effect contributes to a stronger increase in the CO2 flow due to the H+-induced decomposition of bicarbonate moieties. In Fig. 5, desorption curves of water together with nBu-PEI-0.75 and nBu-PEI-1.0 are shown in correlation to the temperature of the solution.
 | ||
| Fig. 5 Desorption flows of nBu-PEI-0.75, nBu-PEI-1.0 and water in correlation with the temperature of the polymer solution. | ||
The desorption behaviour of the acylated polymers is compared with water as there is only a small difference in the amounts of desorbed CO2 of the acylated compounds compared to the desorption behaviour of water. However, we observed a significant increase of about 20% in the desorption flows of the acylated species. Due to the LCST effect of nBu-PEI-1.0 in CO2 containing solution compared with nBu-PEI-0.75, on the one hand, the decomposition of bicarbonate species started earlier in the desorption process. On the other hand, a lower amount of carbon dioxide was desorbed by nBu-PEI-1.0 (Table 2), which led to similar desorption flows for both acylated species. The entropically driven LCST behaviour of nBu-PEI-0.75 and nBu-PEI-1.0 resulted in a significant decrease in pH and to a clear increase in desorption flows. Thermosensitive polymers, especially acylated branched PEIs, are shown to be versatile “green” tools to face the problem of an efficient carbon dioxide release.
Conclusions
Acylated branched polyethylenimine is the first commercially available polymer that shows thermosensitive behaviour in water and also in CO2 containing aqueous solution. This reversible phase transition called lower critical solution temperature is correlated with a shift in pH and a proton release. If used during a desorption process in a standard CO2 capturing unit, said thermosensitive properties of acylated branched polyethylenimine (nBu-PEI-0.75 and nBu-PEI-1.0) represent a novel and highly efficient “green” tool to generate a proton driven decomposition of bicarbonate. In this context, a significant increase in desorption flow of CO2 is reported within this study.b-PEI is the first commercial available homopolymer which can be used as a novel energy efficient CO2 absorbent in aqueous solutions in a standard post combustion capturing process. Hence, thermosensitive polymers, especially acylated branched PEIs, are shown to be versatile “green” tools to face the problem of an efficient carbon dioxide release.
Acknowledgements
We thank the German Federal Ministry of Education and Research and Clariant for financial support.Notes and references
- T. F. Stocker, D. Qin, G.-K. Plattner, M. M. B. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley, Climate Change 2013, The Physical Science Basis, Intergovernmental Panel on Climate Change, Cambidge University Press, Cambidge, New York, Melbourne, Madrid, Cape Town, Singapore, Sao Paulo, Deli, Mexico City, 2013 Search PubMed.
- B. Metz, O. Davidson, H. de Coninck, M. Loos and L. Maye, Carbon Dioxide Capture and Storage, Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, Sao Paulo, 2005 Search PubMed.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kühn, Angew. Chem., Int. Ed., 2011, 50, 8510–8537 CrossRef CAS PubMed.
- G. Centi and S. Perathoner, Catal. Today, 2009, 148, 191–205 CrossRef CAS PubMed.
- M. W. Lehenmeier, S. Kissling, P. T. Altenbuchner, C. Bruckmeier, P. Deglmann, A.-K. Brym and B. Rieger, Angew. Chem., Int. Ed., 2013, 52, 9821–9826 CrossRef CAS PubMed.
- C. E. Anderson, S. I. Vagin, M. Hammann, L. Zimmermann and B. Rieger, ChemCatChem, 2013, 5, 3269–3280 CrossRef CAS.
- C. E. Anderson, S. I. Vagin, W. Xia, H. Jin and B. Rieger, Macromolecules, 2012, 45, 6840–6849 CrossRef CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- G. T. Rochelle, Science, 2009, 325, 1652–1654 CrossRef CAS PubMed.
- P. D. Vaidya and E. Y. Kenig, Chem. Eng. Technol., 2007, 30, 1467–1474 CrossRef CAS.
- K. Goto, K. Yogo and T. Higashii, Appl. Energy, 2013, 111, 710–720 CrossRef CAS PubMed.
- Z. Zhang, Y. Zhao, Q. Gong, Z. Li and J. Li, Chem. Commun., 2013, 49, 653–661 RSC.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2011, 112, 724–781 CrossRef PubMed.
- A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2011, 51, 1438–1463 CrossRef.
- W. Lu, J. P. Sculley, D. Yuan, R. Krishna, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 7480–7484 CrossRef CAS PubMed.
- X. Zhang, X. Zhang, H. Dong, Z. Zhao, S. Zhang and Y. Huang, Energy Environ. Sci., 2012, 5, 6668–6681 CAS.
- S. Lin and P. Theato, Macromol. Rapid Commun., 2013, 34, 1118–1133 CrossRef CAS PubMed.
- P. Schattling, I. Pollmann and P. Theato, React. Funct. Polym., 2014, 75, 16–21 CrossRef CAS PubMed.
- R. H. Weiland, J. C. Dingman and D. B. Cronin, J. Chem. Eng. Data, 1997, 42, 1004–1006 CrossRef CAS.
- Y. Yang, A. J. Mijalis, H. Fu, C. Agosto, K. J. Tan, J. D. Batteas and D. E. Bergbreiter, J. Am. Chem. Soc., 2012, 134, 7378–7383 CrossRef CAS PubMed.
- D. Han, X. Tong, O. Boissière and Y. Zhao, ACS Macro Lett., 2011, 1, 57–61 CrossRef.
- Y. Hoshino, K. Imamura, M. Yue, G. Inoue and Y. Miura, J. Am. Chem. Soc., 2012, 134, 18177–18180 CrossRef CAS PubMed.
- M. Yue, Y. Hoshino, Y. Ohshiro, K. Imamura and Y. Miura, Angew. Chem., Int. Ed., 2014, 53, 2654–2657 CrossRef CAS PubMed.
- H. Kim, S. Lee, M. Noh, S. H. Lee, Y. Mok, G.-w. Jin, J.-H. Seo and Y. Lee, Polymer, 2011, 52, 1367–1374 CrossRef CAS PubMed.
- Y. Furusho and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3404–3411 CrossRef CAS.
- T. C. Drage, K. M. Smith, A. Arenillas and C. E. Snape, Energy Procedia, 2009, 1, 875–880 CrossRef CAS PubMed.
- J. Zhao, F. Simeon, Y. Wang, G. Luo and T. A. Hatton, RSC Adv., 2012, 2, 6509–6519 RSC.
- W. Wang, J. Xiao, X. Wei, J. Ding, X. Wang and C. Song, Appl. Energy, 2014, 113, 334–341 CrossRef CAS PubMed.
- A. Goeppert, M. Czaun, R. B. May, G. K. S. Prakash, G. A. Olah and S. R. Narayanan, J. Am. Chem. Soc., 2011, 133, 20164–20167 CrossRef CAS PubMed.
- BASF, http://www.basf.com/group/corporate/de_DE/brand/LUPASOL, accessed 17.08.2014.
- D. R. Holycross and M. Chai, Macromolecules, 2013, 46, 6891–6897 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectrometry of the acylation reaction, the reactor setup together with a absorption and desorption measurement. See DOI: 10.1039/c4ra13710h |
| This journal is © The Royal Society of Chemistry 2015 |

