A triphenylene-based conjugated microporous polymer: construction, gas adsorption, and fluorescence detection properties†
Jie Han*,
Xia Fan,
Zhan-Zhong Zhuang,
Wei-Chao Song,
Ze Chang*,
Ying-Hui Zhang* and
Xian-He Bu
Department of Chemistry, Tianjin Key Lab on Metal & Molecule-Based Material Chemistry, Key Laboratory of Advanced Energy Materials Chemistry (MOE), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China. E-mail: hanjie@nankai.edu.cn; changze@nankai.edu.cn; zhangyhi@nankai.edu.cn
First published on 27th January 2015
Abstract
A triphenylene-based conjugated microporous polymer (TP-CMP) has been synthesized and shows high surface area (SBET = 1104 m2 g−1) and remarkable uptakes of H2 (7.08 mmol g−1 at 77 K and 900 mmHg) and CO2 (3.37 mmol g−1 at 273 K and 900 mmHg). In addition, it could be utilized to detect nitrobenzene selectively via fluorescence quenching.
Conjugated microporous polymers (CMPs) have many intrinsic advantages such as large specific surface area, high stability, easy functionalization and low skeleton density, which makes them good candidates as potential functional materials in the fields of gas storage and separations,1–5 and heterogeneous catalysis.6 In general, the building units play a crucial role in designing and controlling the structures and properties of CMPs. To date, a number of building units, for example 1,3,5-tribromobenzene,7,8 1,2,4,5-tetrabromobenzene,8 1,3,5-tris(4-bromophenyl)benzene,9 hexa(4-bromophenyl)benzene,10 1,3,5-tri(9-carbazolyl)benzene,11 chloroiodosilole,12 sprio-bis(2,5-dibromopropylenedioxythiophene),13 tris(4-bromophenyl)amine,14 tetrakis(4-bromophenyl)methane,15 tetrakis(4-cyanophenyl)methane,16,17 2,6,14-tribromo-tritycene,18 and triphenylene unit19–22 have been utilized to construct various CMPs for the purpose of exploring basic structure–property relationships as well as practical applications.
Among the various organic units with different structure and properties, triphenylene has been widely used in the preparation of luminescent and semiconducting optoelectronic device based on its rich optical and electronic properties.21,23–25 However, triphenylene is less reported in the research of CMPs compared to the other synthons. Considering about its rigid planar structure with large π system, CMPs composed with this motif could be expected to show high surface area and luminescent property, which is desired for gas sorption and fluorescent detecting applications. Aiming at the exploration of functional CMPs, we focused on the construction and properties investigation of triphenylene based CMPs. Herein, we report a new triphenylene-based CMPs, namely TP-CMP (Scheme 1). As expected, the TP-CMP has a large BET surface area up to 1104 m2 g−1 and can effectively adsorb H2 (7.08 mmol g−1 at 900 mmHg and 77 K) and CO2 (3.37 mmol g−1 at 900 mmHg and 273 K), respectively. Furthermore, TP-CMP exhibits strong fluorescence emission, which can be utilized to the selective detection of nitrobenzene in DMF solution.
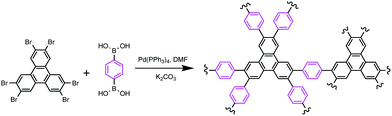
The synthetic route to TP-CMP is depicted in Scheme 1. The detailed procedures are described in the ESI.† To realize the porous structure of triphenylene-based CMPs, 2,3,6,7,10,11-hexabromotriphenylene and 1,4-phenylenebisboronic acid were selected as reactants and connected through a phenyl–phenyl coupling reaction. The formation of TP-CMP can be certified by the Fourier transform infrared (FTIR) spectroscopy and solid state 13C/MAS NMR measurement. The absence of C–Br (511 cm−1) and C–B (1343 cm−1) stretching peak in the FTIR spectra (ESI, Fig. S1†) indicates that the functional Br atoms as well as boronic acid in the reactant have been consumed by phenyl–phenyl coupling. This coupling was further testified by 13C/MAS spectra for TP-CMP as shown in Fig. 1(a), where two broad C sp2 peaks observed at 138.4 and 127.3 ppm can be assigned to the substituted phenyl carbons a (a′ and a′′) and unsubstituted phenyl carbons b (b′), respectively. Similar 13C CP/MAS NMR spectrum have also been reported for polyphenylene-based conjugated microporous polymers.9,35 Thermogravimetric analysis (ESI, Fig. S2a†) was carried out to investigate the structural stability of TP-CMP under nitrogen atmosphere. The profile shows that there is only less than 5% weight loss before 577 °C, demonstrating that it is exceptionally thermally stable. In DSC measurement (Fig. S2b†), no distinct glass transition was observed for TP-CMP below the thermal decomposition temperature owing to the nature of the crosslinking structure, which is consistent with the good physicochemical robustness for most CMPs.
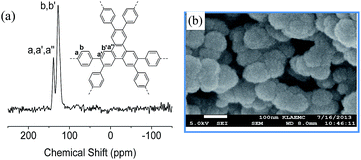 | ||
| Fig. 1 (a) 13C CP/MAS NMR spectra of TP-CMP and (b) scanning electron microscope image of TP-CMP with the scale bar standing for 100 nm. | ||
As seen in Fig. 1(b), the scanning electron micrograph shows that TP-CMP consists of relatively uniform solid submicrometer spheres packing in non-ordered way, resulting in an amorphous structure, due probably to the kinetic control of the reaction. The amorphous structure is also proved by the powder X-ray diffraction pattern (ESI, Fig. S3†), where no PXRD signals of ordered structure are observed.
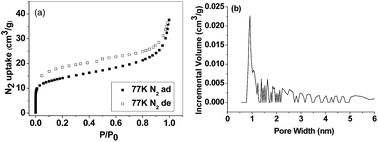
The porosity of the polymer network was investigated by N2 sorption analysis. The N2 isotherm shows type II characteristic with H3 hysteresis loops (Fig. 2(a)), indicating a permanent microporous nature of TP-CMP. Brunauer–Emmett–Teller (BET) calculation gives specific surface area for TP-CMP as 1104 m2 g−1, which is larger than that of 868 m2 g−1 for the covalent organic framework consisting of triphenylene and pyrene moieties,21 and comparable to that of 1148 m2 g−1 for the hexaphenyl benzene-based porous organic polymer.11 Fig. 2(b) shows the pore size distribution (PSD) curve for TP-CMP as calculated using nonlocal density functional theory (NL-DFT), which indicates a microporous diameter narrowly centering around 0.9 nm that localized in the desired pore size range of 0.7–1.2 nm for H2 adsorption.36
It has been predicted theoretically that aromatic units of CMPs could facilitate the adsorption of H2.38 To investigate the hydrogen storage ability of TP-CMP, hydrogen adsorption experiments were performed at 77 K and 87 K, respectively. As shown in Fig. 3, TP-CMP can adsorb 7.08 mmol g−1 H2 at 77 K under 900 mmHg and 5.17 mmol g−1 H2 at 87 K under 900 mmHg, respectively, which is better or comparable to the reported CMPs with similar surface area.10,18,36 The isosteric heats of adsorption (Qst), calculated from the Clausius–Clapeyron equation fitted to the adsorption isotherms of H2 adsorption measured at 77 K, is estimated to be as high as 11.8 kJ mol−1 at zero coverage (see Fig. S4†), which approximates to the ideal adsorption heat for H2 adsorption (15–20 kJ mol−1).39 Based on the structure characterization of TP-CMP, the relatively high H2 uptake of TP-CMP should be attributed to the remarkable larger BET surface and proper pore size, which benefit the enhancement of interaction between H2 and TP-CMP.
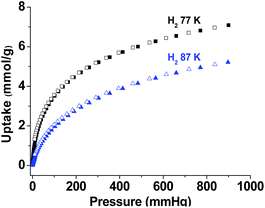 | ||
| Fig. 3 Volumetric H2 adsorption isotherms and desorption isotherms up to 900 mmHg at 77 K and 87 K (adsorption branch is labeled with filled symbols and desorption is labeled with empty). | ||
Motivated by its well performance on H2 adsorption, we test the adsorption isotherms of TP-CMP for CO2, CH4 and N2 at 273 and 298 K (Fig. 4). TP-CMP was observed to exhibit pronounced CO2 uptake and low CH4 and N2 adsorption. The CO2 uptake measured under 900 mmHg is 3.37 mmol g−1 at 273 K and 1.90 mmol g−1 at 293 K, which is larger than or comparable with that of many other CMPs.5,8,19,36,37 The adsorption heat derived from the Clausius–Clapeyron equation fitted to the CO2 adsorption isotherms is 28.7 kJ mol−1 at zero coverage (see Fig. S5†). The selectivity of TP-CMP for CO2 over N2 was calculated by the ideal adsorption solution theory (IAST)40 at a typical composition of 15% CO2 and 85% N2 (Fig. S6†). The obtained CO2/N2 selectivity at 273 K, 23.5–25.2, is comparable with or larger than the corresponding value of many other CMPs.5,41
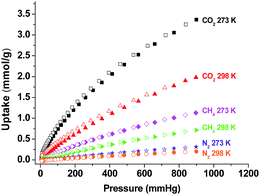 | ||
| Fig. 4 The adsorption and desorption isotherms of CO2, CH4, and N2 at 273 K and 298 K (adsorption branch is labeled with filled symbols and desorption is labeled with empty). | ||
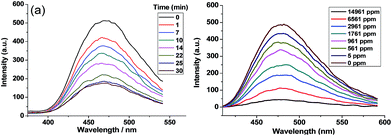
On the other hand, as one of the most common nitroarenes, nitrobenzene is a basic constituent of nitroaromatic explosives as well as notorious environmental pollutant, and thereby could bring about great threat to public safety and health.26,27 In this sense, it is very necessary to develop efficient methods to detect nitrobenzene. In addition, the method to detect nitrobenzene also can be applied to sense other nitroaromatic explosives such as TNT28–32 because of their similarity with each other in nature. It has been verified that conjugated organic molecules and polymers may effectively sense nitroaromatic compounds through weak π–π interaction indicated by spectroscopic methods.33,34 Since the containing of triphenylene motif in the TP-CMP promises its potential as luminescent material, the ability of TP-CMP to act as a nitrobenzene sensor was studied in the solid state according to similar procedures reported in literature.28–31 As expected, the as synthesized CP-CMP shows a emission band centered at 500 nm with high intensity. When exposed to a saturated nitrobenzene atmosphere, it is observed that the emission intensity of TP-CMP is quenched about 18% within 1 min and about 85% within 30 min at room temperature (Fig. 5(a)). These results reveal that the solid TP-CMP is fluorescent response to the vapor of nitrobenzene, which could be utilized for the sensing of nitrobenzene in vapor state. Furthermore, in order to investigate the sensing sensitivity of TP-CMP towards nitrobenzene, a batch of suspensions of TP-CMP dispersed in DMF were prepared with gradually increasing nitrobenzene contents, and their emission spectra were recorded. As shown in Fig. 5(b), the emission intensity show a dramatic decrease even when the concentration of nitrobenzene was as low as 5 ppm. The fluorescence was significantly quenched by 50% at 1761 ppm, and was almost quenched totally by further increasing the concentration of nitrobenzene to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961 ppm. The existence of other solvents has no obvious disturbance to this quenching processes (Fig. S7†), indicating the high selectivity of the sensing process.
961 ppm. The existence of other solvents has no obvious disturbance to this quenching processes (Fig. S7†), indicating the high selectivity of the sensing process.
Conclusions
In conclusion, we have successfully synthesized a porous conjugated microporous polymer TP-CMP derived from triphenylene unit. This compound reveals high surface area and luminescent intensity originated from the triphenylene motif included, which makes it a good candidate for H2 and CO2 storage and fluorescent detection of nitrobenzene. Further investigation on the construction of functional CMPs based on triphenylene is ongoing in our lab.Acknowledgements
This work was financially supported by the 973 Program of China (2012CB821700), National Natural Science Foundation of China (no. 21421001, 21272130 and 21202088) and MOE Innovation Team (IRT13022) of China.Notes and references
- Z. Chang, D.-S. Zhang, Q. Chen and X.-H. Bu, Phys. Chem. Chem. Phys., 2013, 15, 5430–5442 RSC.
- W. C. Song, X.-K. Xu, Q. Chen, Z.-Z. Zhuang and X.-H. Bu, Polym. Chem., 2013, 4, 4690 RSC.
- E. Preis, C. Widling, G. Brunklaus, J. Schmidt, A. Thomas and U. Scherf, ACS Macro Lett., 2013, 2, 380 CrossRef CAS.
- J. L. Novotney and W. R. Dichtel, ACS Macro Lett., 2013, 2, 423 CrossRef CAS.
- R. Dawson, E. Stockel, J. R. Holst, D. J. Adams and A. I. Cooper, Energy Environ. Sci., 2011, 4, 4239 CAS.
- (a) Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083 RSC; (b) C.-J. Liu, Y. Zhao, Y. Li, D.-S. Zhang, Z. Chang and X.-H. Bu, ACS Sustainable Chem. Eng., 2014, 2, 3 CrossRef CAS.
- D.-P. Liu, Q. Chen, Y.-C. Zhao, L.-M. Zhang, A.-D. Qi and B.-H. Han, ACS Macro Lett., 2013, 2, 522 CrossRef CAS.
- Y. Xu and D. Jiang, Chem. Commun., 2014, 50, 2781 RSC.
- H. Ren, T. Ben, F. Sun, M. Guo, X. Jing, H. Ma, K. Cai, S. Qiu and G. Zhu, Mater. Chem., 2011, 21, 10348 RSC.
- C. Zhang, Y. Liu, B. Li, B. Tan, C.-F. Chen, H.-B. Xu and X.-L. Yang, ACS Macro Lett., 2012, 1, 190 CrossRef CAS.
- Q. Chen, M. Luo, P. Hammershøj, D. Zhou, Y. Han, B. W. Laursen, C.-G. Yan and B.-H. Han, J. Am. Chem. Soc., 2012, 134, 6084 CrossRef CAS PubMed.
- W. Shu, C. Guan, W. Guo, C. Wang and Y. Shen, Mater. Chem., 2012, 22, 3075 RSC.
- J.-X. Jiang, A. Laybourn, R. Clowes, Y. Z. Khimyak, J. Bacsa, S. J. Higgigins, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 7577 CrossRef CAS.
- L. Zhang, T. Lin, X. Pan, W. Wang and T.-X. Liu, J. Mater. Chem., 2012, 22, 9861 RSC.
- T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457 CrossRef CAS PubMed.
- D.-S. Zhang, Z. Chang, Y.-B. Lv, T.-L. Hu and X.-H. Bu, RSC Adv., 2012, 2, 408 RSC.
- H. Ren, T. Ben, E. Wang, X. Jing, M. Xue, B. Liu, Y. Cui, S. Qiu and G. Zhu, Chem. Commun., 2010, 46, 291 RSC.
- Q. Chen, M. Luo, T. Wang, J.-X. Wang, D. Zhou, Y. Han, C.-S. Zhang, C.-G. Yan and B.-H. Han, Macromolecules, 2011, 44, 5573 CrossRef CAS.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875 CrossRef CAS PubMed.
- S. S. Han, H. Furukawa, O. M. Yaghi and W. A. Goddard, J. Am. Chem. Soc., 2008, 130, 11580 CrossRef CAS PubMed.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2008, 47, 8826 CrossRef CAS PubMed.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef PubMed.
- E. Tritto, R. Chico, G. Sanz-Enguita, C. L. Flocia, J. Ortega, S. Coco and P. Espinet, Inorg. Chem., 2014, 53, 3449 CrossRef CAS PubMed.
- N. J. Lee, J. H. Jeon, I. In, J. H. Lee and M. C. Suh, Dyes Pigm., 2014, 101, 221 CrossRef CAS PubMed.
- Y. F. Wang, C. X. Zhang, H. Wu and J. L. Pu, J. Mater. Chem. C, 2014, 2, 1667 RSC.
- S. Singh, J. Hazard. Mater., 2007, 144, 15 CrossRef CAS PubMed.
- S. A. Boyd, G. Sheng, B. J. Teppen and C. T. Johnston, Environ. Sci. Technol., 2001, 35, 4227 CrossRef CAS.
- Z. Hu, S. Pramanik, K. Tan, C. Zheng, W. Liu, X. Zhang, Y. J. Chabal and J. Li, Cryst. Growth Des., 2013, 13, 4204 CAS.
- M. Kumar, V. Vij and V. Bhalla, Langmuir, 2012, 28, 12417 CrossRef CAS PubMed.
- A. I. Costa, H. D. Pinto, L. F. V. Ferreira and J. V. Prata, Sens. Actuators, B, 2012, 161, 702 CrossRef CAS PubMed.
- R. S. Dudhe, J. Sinha, A. Kumar and V. R. Rao, Sens. Actuators, B, 2010, 148, 158 CrossRef CAS PubMed.
- G.-Y. Wang, L.-L. Yang, Y. Li, H. Song, W.-J. Ruan, Z. Chang and X.-H. Bu, Dalton Trans., 2013, 42, 12865 RSC.
- T. L. Dunlho, B. J. Laughlin, A. A. Buelt, W. F. Baker, C. A. Conrad and R. C. Smith, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1487 CrossRef.
- P. Beyazkilic, A. Yildirin and M. Bayindir, ACS Appl. Mater. Interfaces, 2014, 6, 4997 CAS.
- J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334 CrossRef CAS PubMed.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012 RSC.
- D. Tian, H.-Z. Zhang, D.-S. Zhang, Z. Chang, J. Han, X.-P. Gao and X.-H. Bu, RSC Adv., 2014, 4, 7506 RSC.
- M. Wong, B. E. Van Kuiken, C. Buda and B. D. Dunietz, J. Phys. Chem. C, 2009, 113, 12571 CAS.
- H. Zhao, Z. Jin, H. Su, J. Zhang, X. Yao, H. Zhao and G. Zhu, Chem. Commun., 2013, 49, 2780 RSC.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121 CrossRef CAS.
- S. Hug, M. B. Mesch, H. Oh, N. Popp, M. Hirsher, J. Senker and B. V. Lotsch, J. Mater. Chem. A, 2014, 2, 5928 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis procedures, IR and TGA measurement, powder X-ray diffraction, adsorption enthalpy of H2 and CO2, adsorption selectivity of CO2 over N2, and fluorescence intensity ratio histograms of TP-CMP in different solvents. See DOI: 10.1039/c4ra13696a |
| This journal is © The Royal Society of Chemistry 2015 |