A novel pH-responsive POSS-based nanoporous luminescent material derived from brominated distyrylpyridine and octavinylsilsesquioxane†
Wenyan Yanga,
Xuesong Jiang*b and
Hongzhi Liu*ac
aKey Laboratory of Special Functional Aggregated Materials, Ministry of Education, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, P. R. China. E-mail: liuhongzhi@sdu.edu.cn; Fax: +86 531 88364691
bSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240, China
cKey Laboratory of Specially Functional Polymeric Materials and Related Technology, Ministry of Education, East China University of Science and Technology, Shanghai, 200237, China
First published on 16th January 2015
Abstract
For the first time, a novel pH-responsive porous material based on POSS was easily prepared from the cubic octavinylsilsesquioxane (OVS) and brominated distyrylpyridine (Br-DSP) via the Heck coupling reaction. The resulting material possessed high porosity with Brunauer–Emmett–Teller specific surface area of 600 m2 g−1 and total pore volume of 0.58 cm3 g−1. It also exhibited high CO2 storage capability of 0.82 mmol g−1 (3.62 wt%) at 298 K/760 Torr. The porous polymer was luminescent with the maximum emission at ca. 530 nm in the solid state, due to the incorporation of the conjugated structures of distyrylpyridine. Owing to the protonated nitrogen centers in the porous material, an excellent pH-responsive property was observed and a linear relation was established between the maximum luminescent emission wavelengths (λem) of the turbid liquid in buffer solutions and the corresponding pH values in the pH range from 1 to 4. This porous material is very promising as a fluorescent probe in the rapid test systems.
Introduction
Porous materials with novel intrinsic properties such as high specific surface area, excellent porosity and good thermal stability have potential applications in gas storage and separation,1 optical devices,2–4 heterogeneous catalysis,5 etc. Covalently-linked porous polymers have attracted specific interest because their structures and properties are easily tuned through rational design.6 By selecting the ideal building blocks and appropriate chemical reactions, an excellent porous polymer can be efficiently produced. Usually, functional building blocks with rigid structures are selected to form stable pores in the crosslinked polymer, which prevent the skeleton from collapsing.7 In addition, the functional building blocks could endow the porous material with many novel properties.7–9Recently, functional fluorescent porous polymers with intrinsic fluorescence and three-dimensional (3D) network structures10,11 have been extensively investigated. Some of these polymers were successfully prepared by selecting monomers with rigid, contorted structures and π-electronic conjugated components,10–14 such as spirobifluorene,2,15 phenyl,16 carbazole,17 pyrene,18 and tetraphenylethene19,20 units.
Polyhedral oligomeric silsesquioxanes (POSS), with a rigid 3D nanometer-sized inorganic–organic hybrid structures are particularly suitable building blocks to construct porous polymers.21,22 Many porous polymers derived from POSS have been prepared via polymerizations reactions including hydrosilylation,23 Sonogashira coupling reaction,24 Yamamoto coupling reaction,25 Heck reaction26,27 and Friedel–Crafts reaction,28–30 radical polymerization31,32 and others.33,34 By utilizing their rigid and bulky cages, POSS can increase the quantum yields of fluorescent molecules or polymers due to their enhanced aggregation prevention of dye molecules and the inhibition of self-quenching in the solid state.35–37 However, POSS-based luminescent porous materials have rarely been investigated so far, and the development of novel fluorescent porous polymers based on POSS could be a promising field in the future.
The design and application of various sensing porous materials, which respond to outer stimulus such as changes in pH, luminosity and temperature, have become one of the focuses in the field of environmentally, biologically and medically oriented chemical analysis.38 Some pH responsive porous materials such as porous glass,39 hydrogel-capped porous SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and nanoporous silica colloidal membranes have been reported.41 However, up to date, all of the pH responsive porous materials were obtained by physically absorbing the pH responsive molecules.42–46 The resulting interactions were relatively weak and the responsive molecules easily fell off from the porous materials. Therefore, it was imperative to develop a novel pH responsive porous polymer where the responsive molecules are covalently-linked within the materials matrix.
40 and nanoporous silica colloidal membranes have been reported.41 However, up to date, all of the pH responsive porous materials were obtained by physically absorbing the pH responsive molecules.42–46 The resulting interactions were relatively weak and the responsive molecules easily fell off from the porous materials. Therefore, it was imperative to develop a novel pH responsive porous polymer where the responsive molecules are covalently-linked within the materials matrix.
Distyrylpyridine (DSP) analogs are pyridine derivatives with structurally fixed styryl moieties and they are a novel class of fluorescence materials.47 Their structural rigidity would endow them with high fluorescence quantum yields and nitrogen atoms in the pyridine moieties would be potential protonated centers. These characteristics make them potential sensing materials because the protonation of the rather effective fluorophores leads to pronounced changes in fluorescence spectra.48–50 Some excellent pH-sensing materials based on DSP derivatives have been prepared through the non-covalent immobilization of these dyes on silica. Due to the insolubility of DSPs in water, they could be applied in the analysis of aqueous media. However, these dyes probably fell off from silica while performing the analysis of organic media.
Considering the structural rigidity of DSP, some novel functional DSP derivatives could be designed and synthesized and they could act as rigid building blocks to construct covalently-linked porous materials to overcome the above-mentioned limitations through non-covalent adsorption.51–53 In this paper, for the first time, we have synthesized brominated distyrylpyridine (Br-DSP) through one-step reaction, which was confirmed by NMR, FTIR and mass spectrum (ESI†).
It was expected that this novel fluorescent molecule (Br-DSP) could react with octavinylsilsesquioxane (OVS) to construct hybrid nanoporous polymer via the Heck reaction. Thus, the distyrylpyridine rigid structure was covalently incorporated into POSS-based porous material. This hybrid porous polymer (HPP) is hopeful to keep the excellent pH-sensing property and has promising and wide applications in the rapid detecting system.
Experimental section
Materials
Unless otherwise noted, all reagents were purchased from commercial suppliers and used without further purification. Octavinylsilsesquioxane (OVS) was synthesized as the previous reports.54,55 Br-DSP was synthesized through one-step reaction according to our previous report.47 N,N-dimethylformamide (DMF) was dried over CaH2 at 80 °C for 12 h and distilled under vacuum pressure, then stored with 4 Å molecule sieves prior to use. Triethylamine (Et3N) was dried by distillation over CaH2 and used freshly.Measurements
Fourier-transform infrared (FT-IR) spectra of the products were recorded on a Bruker Tensor 27 spectrophotometer from 4000 to 400 cm−1. Solid-state 29Si MAS NMR and 13C CP/MAS NMR spectra were performed on a Bruker AVANCE-500 NMR Spectrometer operating at the resonance frequencies of 125 MHz for 13C and 99 MHz for 29Si under a magnetic field strength of 9.4 T. A chemagnetics of 5 mm triple-resonance MAS probe was used to acquire 13C and 29Si NMR spectra. 29Si MAS NMR spectra with high power proton decoupling were recorded using a π/2 pulse length of 5 μs, a recycle delay of 120 s and a spinning rate of 5 kHz. Elemental analyses were conducted by using an Elementarvario EL III elemental analyzer. The pH of the buffer solutions was controlled by the universal pH meter PHS-3C with the glass electrode ESL-11g-05, which was calibrated by the standard aqueous buffer solutions (Leici, Shanghai). Luminescence (excitation and emission) spectra were determined with a Hitachi F-4500 spectrophotometer.Field-emission scanning electron microscopy (FE-SEM) tests were performed by using a HITACHI S4800 spectrometer. The high-resolution transmission electron microscopy (HR-TEM) experiments were recorded on a JEM 2100 electron microscope (JEOL, Japan) with an acceleration voltage of 200 kV. Thermal gravimetric analyses (TGA) were measured on a Mettler-Toledo TGA/DSC system under nitrogen at 10 °C min−1 to 800 °C. Powder X-ray diffraction (PXRD) were performed on a Riguku D/MAX 2550 diffractometer with Cu-Kα radiation, 40 kV, 200 mA with the 2θ range of 10° to 80° at room temperature.
Nitrogen sorption isotherm measurements were recorded by using a Micro Meritics surface area and pore size analyzer. The samples were degassed at 160 °C for 12 h prior to measurement. A sample of ca. 0.1 g and a UHP-grade nitrogen (99.999%) gas source were selected in the nitrogen sorption measurements at 77 K and collected on a Quantachrome Quadrasorb apparatus. BET surface areas were evaluated at a P/P0 range of 0.01 to 0.20. Nonlocal density functional theory (NL-DFT) pore size distributions were performed on the carbon/slit-cylindrical pore mode of the Quadrawin software.
Synthesis of hybrid porous polymers (HPP)
An oven-dried flask was added OVS (211 mg, 0.33 mmol), Br-DSP (576 mg, 0.89 mmol), palladium acetate (34 mg, 0.15 mmol) and tris(2-methylphenyl)phosphine (91 mg, 0.30 mmol) in DMF/Et3N (45 mL/15 mL) under argon. The mixture was bubbled by argon under stirring for 30 min at room temperature and then heated at 100 °C for 48 h. After cooling to room temperature, the mixture was filtered and washed with water, THF, methanol, chloroform and acetone to remove the remnant amine salts, residuary monomers and catalyst. The products were further purification under the Soxhlet extractor with THF for 24 h and methanol for 24 h, and dried in vacuo at 70 °C for 48 h. HPP was afforded as a Kelly solid (578 mg). Yield: 101%. Elemental analysis calc. (wt%) for Si24O36C296H224N8: C 69.13, H 4.39, N 2.18. Found C 63.52, H 5.07, N 2.14.Results and discussion
As shown in Scheme 1, HPP was prepared through the Heck reaction of OVS and Br-DSP in N,N-dimethylformamide (DMF) at 100 °C for 48 h. A highly crosslinking network was formed after the cross-coupling reaction, which favoured formation of pores and the resulting hybrid materials did not dissolve in most common solvents, for example, THF, chloroform, methanol and ethanol etc. Unlike non-covalent immobilization of DSP dyes on silica, these dye units keep stable in the network while performing the analysis of organic media.The structure of HPP was confirmed by FTIR, solid-state 13C cross polarization/magic angle spinning (CP/MAS) NMR and 29Si MAS NMR spectroscopy. FTIR spectrum of HPP showed that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration peaks of ethenylene and aryl groups for HPP were observed at 1412, 1510 and 1601 cm−1; the strong peak at 1124 cm−1 was assigned to the –Si–O–Si– stretching vibrations, which confirmed the existing POSS cages in HPP (Fig. 2S†).56 There existed C
C stretching vibration peaks of ethenylene and aryl groups for HPP were observed at 1412, 1510 and 1601 cm−1; the strong peak at 1124 cm−1 was assigned to the –Si–O–Si– stretching vibrations, which confirmed the existing POSS cages in HPP (Fig. 2S†).56 There existed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, –Si–O–Si– and aryl groups simultaneously, indicating the presence of cross-linking networks in the hybrid polymer.
C, –Si–O–Si– and aryl groups simultaneously, indicating the presence of cross-linking networks in the hybrid polymer.
The solid-state 29Si NMR spectrum of HPP showed signals at −62.4, −68.2 and −79.9 ppm, respectively (Fig. 1), which were attributed to the silicon atoms of T1, T2 and T3 units (Tn: CSi(OSi)n(OH)3−n) in the resulting network. T3 unit arose from the unreacted Si–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 units and the resulting Si–CH
CH2 units and the resulting Si–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Ar– units after the Heck reaction. The presence of T1 and T2 units indicated that partial POSS cages cleaved during the synthesis. This could be caused by three reasons. First, excessive Et3N was added as the acid absorbent in the Heck reaction, which could rupture the siloxane bonds. Second, HBr was formed during the synthesis. Although acid absorbent was added, the resulting HBr still may be a potential factor to cleave POSS cages. The third factor is that the rigid or straight structures could cause partial collapse of the POSS cages during the release of structural stress in the network. This result confirmed the existence of intact POSS moieties and partial POSS cages distorted, which was accorded with other porous polymers derived from POSS.25,57,58 Nevertheless, nearly 70% of POSS cages were remained intact according to the relative intensity ratios of T3 signal, T3/(T1 + T2 + T3) = 0.697.
CH–Ar– units after the Heck reaction. The presence of T1 and T2 units indicated that partial POSS cages cleaved during the synthesis. This could be caused by three reasons. First, excessive Et3N was added as the acid absorbent in the Heck reaction, which could rupture the siloxane bonds. Second, HBr was formed during the synthesis. Although acid absorbent was added, the resulting HBr still may be a potential factor to cleave POSS cages. The third factor is that the rigid or straight structures could cause partial collapse of the POSS cages during the release of structural stress in the network. This result confirmed the existence of intact POSS moieties and partial POSS cages distorted, which was accorded with other porous polymers derived from POSS.25,57,58 Nevertheless, nearly 70% of POSS cages were remained intact according to the relative intensity ratios of T3 signal, T3/(T1 + T2 + T3) = 0.697.
The Solid-state 13C CP/MAS NMR spectra of HPP and Br-DSP were also analyzed in this work (Fig. 2). Compared with Br-DSP, the ethenylene units of HPP were formed through Heck reactions showed peaks at δ = 128 and 159 ppm. The signals of aryl and unreacted vinyl carbon atoms were observed at δ = 93–142 ppm, and the signals of saturated carbon atoms approached pyridine ring exhibited multiple peaks and displayed at δ = 0–37 ppm, corresponding with the peaks of Br-DSP. The carbon atoms of the pyridine ring were observed at 190 and 196 ppm, which showed shifted to low field because the ethenylene units extending the length of the conjugated system.
The porous properties of HPP were evaluated by nitrogen adsorption and desorption measurements at 77 K. HPP exhibited a sharp uptake at low relative pressures and gradually increasing uptake at higher relative pressures with a inconspicuous hysteresis in Fig. 3, suggesting the presence of micropores and mesopores simultaneously.26,27,29,30 The surface area of HPP was ascertained by the specific Brunauer–Emmett–Teller surface areas (SBET) method. SBET of HPP was calculated as 603 m2 g−1 and the micropore surface area was calculated as 347 m2 g−1 by t-plot method. Pore size distribution of the polymer was confirmed from the nonlocal density functional theory (NL-DFT). The result was accorded with the consequence of the N2 isotherm and indicated the presence of micropores and mesopores within structures. As shown in Fig. 3, HPP manifested the uniform micropores with an average diameter centered at about 1.54 nm and a broad distribution range of mesopores. The total pore volume (Vtotal) of HPP was 0.58 cm3 g−1 and the ratio of the micropore volume (Vmicro) to the Vtotal was 0.27.
The thermal stability of the polymer was evaluated by thermogravimetric analysis (TGA) under N2 at 10 °C min−1 from 45 °C to 800 °C. HPP possessed good thermal stability and the mass remained invariant up to approximately 440 °C (decomposition temperature at 5 wt% mass loss), which was significantly more stable than OVS and Br-DSP due to highly cross-linking networks (Fig. 3S†). The initial mass loss of HPP can be attributed to the breakage of organic moieties such as residual vinyl and phenyl groups. The decomposition that occurred at about 510 °C may be derived from the rupture of the siloxane spacers.26–30,57
Although OVS is highly crystalline, powder X-ray diffraction (PXRD) patterns showed that the HPP was amorphous with no long-range crystallographic order and showed broad diffraction peaks at ∼22° 2θ, which may be assigned to Si–O–Si linkages (Fig. 4S†).26–30,34
The particle size and morphology were observed by field emission scanning electron microscopy (FE-SEM). HPP was shown the structure consisted of inter-linking irregular shapes with a wide size distribution range from 100 nm to tens of micrometers, in accordance with the consequence of PXRD (Fig. 5Sa†).26,27,29 The texture and ordering of HPP was evaluated by high-resolution transmission microscopy (HR-TEM). HR-TEM image confirmed the porous characteristics of HPP with a relatively uniform pore diameter. HPP was stable under the electron beam (Fig. 5Sb†).
Carbon dioxide storage is one of the potentially important applications for porous materials. And some of those porous materials based on POSS have been tested for CO2 storage.30,31,58 It is interesting to investigate the CO2 storage capacity for HPP after the introduction of pyridine groups into the hybrid network based on POSS. As shown in Fig. 4, the CO2 storage capacity for HPP was 0.82 mmol g−1 (3.62 wt%) at 298 K/760 Torr. This result was comparable or higher than the corresponding values of other porous polymers based on POSS with higher SBET than HPP.29,30 This relatively high CO2 uptake capacity might be ascribed to the high affinity towards CO2 through the strong dipole–dipole interaction and acid–base interaction by protonated and deprotonated states of the pyridine rings.59–62
From the above-mentioned results, we know that the simultaneous introduction of the structural rigidity of bulky POSS cage and DSP structure make HPP possess good porosity and thermal stability. Moreover, the possible protonation of the highly basic nitrogen atom of pyridine moiety would result in substantial changes in fluorescence spectra and this feature could be used in the design of new fluorescence pH sensors.
Firstly, it is necessary to know whether the luminescence of HPP changes or not after the introduction of the structure of DSP units into the porous network. Compared with the luminescent spectrum of Br-DSP, HPP exhibited the similar maximum emission wavelength (λem) at ca. 530 nm (Fig. 6S†). It was a typical characteristic of green emitting materials. The emission can be assigned to the π–π* transition including the aryl, ethenylene groups, pyridyl groups in DSP and the POSS cages elicit limited effects on the π systems.27,35,36,63 This indicated that the fluorescent property of HPP derived from the structure of DSP was almost unaffected and some typical properties of DSP could be also utilized for HPP, for example, the remarkable pH response.
In order to investigate the pH-response of HPP, a series of buffer solutions were prepared on the base of the distilled water, acids, bases and salts solutions, i.e. oxalic acid and potassium oxalate (for pH range 1.00–2.50), citric acid and disodium hydrogen phosphate (for pH range 3.00–8.00) and glycocoll and sodium hydroxide (for pH range 8.50–12.00) with the step of 0.50 pH.49,64,65 The initial concentration of these acid, base solutions was 0.1 mol dm−3, the influence of the ion strength in the buffer solution on the fluorescence emission wavelength was negligible in this study.
Photostability is one of the key factors tested for responsive ability. Sensing materials irradiated under continuous ultraviolet light usually result in a weakening fluorescence signal, but several other parameters such as emission band shape and fluorescence lifetime usually remained unchanged. The spectral and protolytic properties of the DSPs could be influenced by undergoing effective E, Z-photoisomerization under ultraviolet irradiation.49 HPP in buffer solution of pH = 7 was irradiated under continuous ultraviolet light (250 W Hg lamp, 365 nm) for 2 h, the fluorescence intensity was decreased about 60%; while the emission spectrum of HPP was unchanged. This implied that the fluorescent signal of HPP for pH test was not affected by UV irradiation (Fig. 7S†).
3 mg HPP was dispersed in 5 mL buffer solutions by the ultrasonic method and the resulting suspension solution was tested by fluorescence spectroscopy. The fluorescence spectra of the suspensions of HPP at different pH values were shown in Fig. 5, the excitation wavelength of 370 nm was chosen. It is clearly observed that remarkable bathochromic shifts of the λem of HPP occurred as pH value changed from 5.5 to 1, which were assigned to the protonation process of HPP.44 The λem of HPP at different pH values were listed in Table 1S,† λem of HPP shifted from 525 to 618 nm when the pH values changed from 5.50 to 1.00. At pH = 5.50, the suspension of HPP exhibited a typical characteristic of green emitting and gradually transformed to red emitting at pH = 1.00. The photos of HPP suspension in the pH range from 1.00 to 5.50 under UV (365 nm) illumination were shown in Fig. 9S.† The light colors were accorded with the corresponding characteristic emissions in fluorescence spectra. However, the λem of HPP almost remained at 524 nm in the pH range from 5.50 to 12.00 (Fig. 10S†). The suspensions of HPP emitted a green light at the pH values from 5.50 to 12.00 under UV-irradiation (365 nm) (Fig. 11S†).
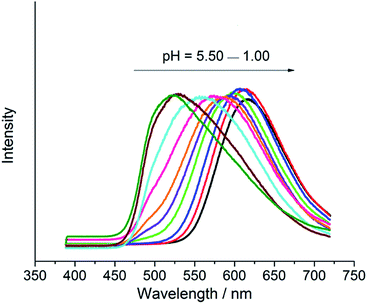 | ||
| Fig. 5 Fluorescence spectra of suspensions of HPP in buffer solutions with the pH in the range from 5.50 to 1.00 with interval of 0.5 pH (excited at 370 nm). | ||
It was supposed that the pH response of HPP should be ascribed to the conversion between protonation and deprotonation states of DSP moieties.66,67 The pKa value for the conjugate acid of pyridine groups were 5.25.66–68 This meant that the pyridyl groups were in the protonated states when pH value was below 5.25 and increasing acidity favoured the protonation of the pyridinic nitrogen atom (Scheme 2).
A good linear correlation between λem and pH was observed in the pH interval between 1 and 4. The correlation coefficient was 0.98 in Fig. 6 and a regression formula of pH = −0.069λem + 44 was established. This indicates that HPP could be applied as a pH indicator for acid solution, for example, gastric acid with a pH range of 1.5 to 3.5. The linear correlation coefficients were 0.96 and 0.89 as the pH values in the range of 1.00–4.50 (Fig. 12S†) and 1.00–5.00 (Fig. 13S†), respectively. The linear correlation coefficients were lower than 0.98 as the pH values in the range of 1.00–4.00, the reason that was the protonation and deprotonation of the pyridine groups coexisted at pH = 4.50–5.50. However, when pH is increased to above 5.25, deprotonation states of pyridyl groups showed no pH response and λem almost remained at 525 nm.
 | ||
| Fig. 6 Plots of the fluorescence maximum emission wavelength of HPP versus pH values (pH = 1.00–4.00). | ||
We selected two buffer solutions at pH = 1, 12 to investigate the recyclability of HPP. First, HPP was immersed in the buffer solution of pH = 1; then the above buffer solution was replaced by the buffer solution of pH = 12. Finally, the buffer solution of pH = 12 was replaced by the buffer solution of pH = 1 again. Each suspension of HPP was tested by fluorescence spectroscopy (Fig. 14S†). The fluorescence spectra of suspensions of HPP confirmed that the protonation and deprotonation of HPP was reversible, indicating that HPP could be recyclable.
Conclusions
In summary, a novel pH-responsive fluorescent nanaoporous polymer has been prepared successfully through the Heck reaction by using octavinylsilsequioxane (OVS) and brominated distyrylpyridine (Br-DSP) with excellent pH-response. The porous polymer exhibited porosity with SBET of 600 m2 g−1 and higher CO2 storage capability of 0.82 mmol g−1 (3.62 wt%) at 298 K/760 Torr. It was particularly worth mentioning that this POSS-based nanoporous polymer exhibited superior pH response, compared with other POSS-based porous materials reported previously. PH values and the corresponding λem have a good linear relation in the pH interval between 1 and 4 (pH = −0.069λem + 44) and the linear correlation coefficient was 0.98. This indicates that this porous polymer is very promising as a fluorescent probe in the rapid test of gastric acid, acidic waste etc.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21274081).Notes and references
- R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4699 CrossRef PubMed.
- Q. Chen, J. Wang, Q. Wang, N. Bian, Z. Li, C. Yan and B. Han, Macromolecules, 2011, 44, 7987 CrossRef CAS.
- J. Schmidt, J. Weber, J. D. Epping, M. Antonietti and A. Thomas, Adv. Mater., 2009, 21, 702 CrossRef CAS.
- G. Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093 CrossRef PubMed.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675 RSC.
- J. X. Jiang, A. Trewin, F. Su, C. D. Wood, H. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, Macromolecules, 2009, 50, 2658 CrossRef.
- D. Wu, F. Xu, R. Sun, R. Fu, H. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959 CrossRef CAS PubMed.
- B. Kiskan, M. Antonietti and J. Weber, Macromolecules, 2012, 45, 1356 CrossRef CAS.
- T. Ben, K. Shi, Y. Cui, C. Pei, Y. Zuo, H. Guo, D. Zhang, J. Xu, F. Deng, Z. Tian and S. Qiu, J. Mater. Chem., 2011, 21, 18208 RSC.
- A. Patra and U. Scherf, Chem.–Eur. J., 2012, 18, 10074 CrossRef CAS PubMed.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012 RSC.
- A. Patra, J. Koenen and U. Scherf, Chem. Commun., 2011, 47, 9612 RSC.
- K. Zhang, B. Tieke, F. Vilela and P. J. Skabara, Macromol. Rapid Commun., 2011, 32, 825 CrossRef CAS PubMed.
- D. Xiao, Y. Li, L. Liu, B. Wen, Z. Gu, C. Zhang and Y. S. Zhao, Chem. Commun., 2012, 48, 9519 RSC.
- J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334 CrossRef CAS PubMed.
- L. Chen, Y. Honsho, S. Seki and D. L. Jiang, J. Am. Chem. Soc., 2010, 132, 6742 CrossRef CAS PubMed.
- X. M. Liu, Y. H. Xu and D. L. Jiang, J. Am. Chem. Soc., 2012, 134, 8738 CrossRef CAS PubMed.
- J. X. Jiang, A. Trewin, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1777 RSC.
- Y. Xu, A. Nagai and D. Jiang, Chem. Commun., 2013, 49, 1591 RSC.
- Q. Chen, J. Wang, F. Yang, D. Zhou, N. Bian, X. Zhang, C. Yan and B. Han, J. Mater. Chem., 2011, 21, 13554 RSC.
- R. M. Laine and M. F. Roll, Macromolecules, 2011, 44, 1073 CrossRef CAS.
- K. Tanaka and Y. Chujo, J. Mater. Chem., 2012, 22, 1733 RSC.
- C. X. Zhang, F. Babonneau, C. Bonhomme, R. M. Laine, C. L. Soles, H. A. Hristov and A. F. Yee, J. Am. Chem. Soc., 1998, 120, 8380 CrossRef CAS.
- J. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 1072 CrossRef CAS PubMed.
- W. Chaikittisilp, A. Sugawara, A. Shimojima and T. Okubo, Chem. Mater., 2010, 22, 4841 CrossRef CAS.
- D. Wang, L. Xue, L. Li, B. Deng, S. Feng, H. Liu and X. Zhao, Macromol. Rapid Commun., 2013, 34, 861 CrossRef CAS PubMed.
- D. Wang, W. Yang, L. Li, X. Zhao, S. Feng and H. Liu, J. Mater. Chem. A, 2013, 1, 13549 CAS.
- W. Chaikittisilp, M. Kubo, T. Moteki, A. Sugawara-Narutaki, A. Shimojima and T. Okubo, J. Am. Chem. Soc., 2011, 133, 13832 CrossRef CAS PubMed.
- Y. Wu, D. Wang, L. Li, W. Yang, S. Feng and H. Liu, J. Mater. Chem. A, 2014, 2, 2160 CAS.
- W. Yang, D. Wang, L. Li and H. Liu, Eur. J. Inorg. Chem., 2014, 2976 CrossRef CAS.
- H. Lin, J. Ou, Z. Zhang, J. Donga and H. Zou, Chem. Commun., 2013, 49, 231 RSC.
- F. Alves and I. Nischang, Chem.–Eur. J., 2013, 19, 17310 CrossRef CAS PubMed.
- N. Shanmugam, K. T. Lee, W. Y. Cheng and S. Y. Lu, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2521 CrossRef CAS.
- M. F. Roll, J. W. Kampf, Y. Kim, E. Yi and R. M. Laine, J. Am. Chem. Soc., 2010, 132, 10171 CrossRef CAS PubMed.
- S. Zheng, H. Phillips, E. Geva and B. D. Dunietz, J. Am. Chem. Soc., 2012, 134, 6944 CrossRef CAS PubMed.
- H. Araki and K. Naka, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4170 CrossRef CAS.
- R. M. Laine, S. Sulaiman, C. Brick, M. Roll, R. Tamaki, M. Z. Asuncion, M. Neurock, J. S. Filhol, C. Y. Lee, J. Zhang, T. Goodson, M. Ronchi, M. Pizzotti, S. C. Rand and Y. Li, J. Am. Chem. Soc., 2010, 132, 3708 CrossRef CAS PubMed.
- F. B. M. Suah, M. Ahmad and M. N. Taib, Sens. Actuators, B, 2003, 90, 182 CrossRef CAS.
- T. Yazawa, S. Miyamoto, S. Yusa, T. Jin and A. Mineshige, Mater. Res. Bull., 2013, 48, 4267 CrossRef CAS PubMed.
- J. Wu and M. J. Sailor, Adv. Funct. Mater., 2009, 19, 733 CrossRef CAS.
- O. Schepelina, N. Poth and I. Zharov, Adv. Funct. Mater., 2010, 20, 1962 CrossRef CAS.
- H. Y. Tang, J. Guo, Y. Sun, B. S. Chang, Q. G. Ren and W. L. Yang, Int. J. Pharm., 2011, 421, 388 CrossRef CAS PubMed.
- R. Liu, P. H. Liao, J. K. Liu and P. Y. Feng, Langmuir, 2011, 27, 3095 CrossRef CAS PubMed.
- H. Zhang, S. Bai, J. Sun, J. Han and Y. Guo, Mater. Res. Bull., 2014, 53, 266 CrossRef CAS PubMed.
- Y. F. Zhu and J. L. Shi, Microporous Mesoporous Mater., 2007, 103, 243 CrossRef CAS PubMed.
- G. W. de Groot, M. G. Santonicola, K. Sugihara, T. Zambelli, E. Reimhult, J. Voros and G. J. Vancso, ACS Appl. Mater. Interfaces, 2013, 5, 1400 CAS.
- X. Jiang, J. Yin, Y. Murakami and M. Kaji, J. Photopolym. Sci. Technol., 2009, 22, 351 CrossRef CAS.
- V. G. Pivovarenko, A. V. Grygorovych, V. F. Valuk and A. O. Doroshenko, J. Fluoresc., 2003, 13, 479 CrossRef CAS.
- O. V. Grygorovych, S. M. Moskalenko, B. A. Marekha and A. O. Doroshenko, J. Fluoresc., 2010, 20, 115 CrossRef CAS PubMed.
- V. F. Valuk, V. G. Pyvovarenko, O. V. Grygorovych and A. O. Doroshenko, Theor. Exp. Chem., 2004, 40, 256 Search PubMed in Russian.
- J. R. Holst, E. Stöckel, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 8531 CrossRef CAS.
- E. Stöckel, X. Wu, A. Trewin, C. D. Wood, R. Clowes, N. L. Campbell, J. T. A. Jones, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Chem. Commun., 2009, 212 RSC.
- D. Yuan, W. Lu, D. Zhao and H. C. Zhou, Adv. Mater., 2011, 23, 3723 CrossRef CAS PubMed.
- J. F. Brown, L. H. Vogt and P. I. Prescott, J. Am. Chem. Soc., 1964, 86, 1120 CrossRef CAS.
- D. Chen, S. Yi, W. Wu, Y. Zhong, J. Liao, C. Huang and W. Shi, Polymer, 2010, 51, 3867 CrossRef CAS PubMed.
- I. Nischang, O. Brüggemann and I. Teasdale, Angew. Chem., Int. Ed., 2011, 50, 4592 CrossRef CAS PubMed.
- W. Chaikittisilp, A. Sugawara, A. Shimojima and T. Okubo, Chem.–Eur. J., 2010, 16, 6006 CrossRef CAS PubMed.
- Y. Peng, T. Ben, J. Xu, M. Xue, X. Jing, F. Deng, S. Qiu and G. Zhu, Dalton Trans., 2011, 40, 2720 RSC.
- Y. Zhao, Q. Cheng, D. Zhou, T. Wang and B. Han, J. Mater. Chem., 2012, 22, 11509 RSC.
- Q. Chen, M. Luo, P. Hammershøj, D. Zhou, Y. Han, B. W. Laursen, C. Yan and B. Han, J. Am. Chem. Soc., 2012, 134, 6084 CrossRef CAS PubMed.
- L. Feng, Q. Chen, J. Zhu, D. Liu, Y. Zhao and B. Han, Polym. Chem., 2014, 5, 3081 RSC.
- D. Wang, L. Li, W. Yang, Y. Zuo, S. Feng and H. Liu, RSC Adv., 2014, 4, 59877 RSC.
- X. Jing, F. Sun, H. Ren, Y. Tian, M. Guo, L. Li and G. Zhu, Microporous Mesoporous Mater., 2013, 165, 92 CrossRef CAS PubMed.
- F. Kobayashi, H. Ikeura, S. Odake, S. Tanimoto and Y. Hayata, LWT–Food Sci. Technol., 2012, 48, 330 CrossRef CAS PubMed.
- Y. C. Chen, R. Xie and L. Y. Chu, J. Membr. Sci., 2013, 442, 206 CrossRef CAS PubMed.
- J. Lindqvist, D. Nyström, E. Östmark, P. Antoni, A. Carlmark, M. Johansson, A. Hult and E. Malmström, Biomacromolecules, 2008, 9, 2139 CrossRef CAS PubMed.
- S. Liu, L. Wang, B. Liu and Y. Song, Polymer, 2013, 54, 3065 CrossRef CAS PubMed.
- B. Yameen, M. Ali, R. Neumann, W. Ensinger, W. Knoll and O. Azzaroni, Nano Lett., 2009, 9, 2788 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure (Br-DSP), and supplementary figures (EDS, FTIR, TGA, PXRD, FE-SEM, HR-TEM and fluorescence spectra of HPP, and 1H NMR, solid-state 13C CP/MAS NMR spectrum and mass spectrum of Br-DSP). See DOI: 10.1039/c4ra13628d |
| This journal is © The Royal Society of Chemistry 2015 |






