Enhanced proton conductivity of proton exchange membrane at low humidity based on poly(methacrylic acid)-loaded imidazole microcapsules
Abstract
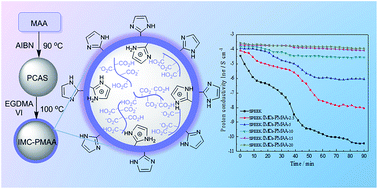
Inspired by the water reserving function of vacuoles in plant cells, poly(methacrylic acid)-loaded imidazole microcapsules (IMCs-PMAA) with high water retention were prepared by distillation–precipitation polymerization and incorporated into a sulfonated poly(ether ether ketone) (SPEEK) matrix to fabricate SPEEK/IMCs-PMAA composite membranes. Compared with the SPEEK control membrane, the composite membranes exhibit higher water uptake, lower swelling degree and better methanol barrier properties. The water retention capacity of the microcapsules is optimized by the carboxylic acid groups of PMAA and the strong electrostatic attraction between the imidazole groups and sulfonic acid groups. Proton transfer pathways are constructed both inside the microcapsules and at the IMCs–SPEEK interface to enhance proton conductivity. The highest proton conductivity of composite membranes is about 5.52 × 10−2 S cm−1 at room temperature and 100% relative humidity (RH), which is more than two times of the SPEEK control membrane conductivity (2.51 × 10−2 S cm−1). In particular, the SPEEK/IMCs-PMAA-20 membrane exhibits the highest proton conductivity of 1.93 × 10−2 S cm−1 at 20% RH, which is two orders of magnitude higher than that of the SPEEK control membrane. The high water retention and proton conduction properties demonstrate that the composite membranes have a great potential application for the direct methanol fuel cell (DMFC).

 Please wait while we load your content...
Please wait while we load your content...