Controllable synthesis and morphology-dependent photocatalytic performance of anatase TiO2 nanoplates†
Abstract
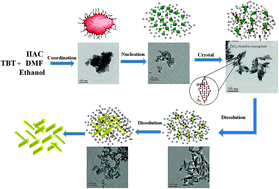
Uniform rhombic anatase TiO2 nanoplates with selectively exposed {010} facets were successfully fabricated using a novel solvothermal method without adding surfactant or templates. In our synthetic approach, the coordination effect of acetic acid (HAc) and N,N-dimethyl formamide (DMF) regulated the reaction rate and contributed to the formation of TiO2 nanoplates. The TiO2 samples were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), infrared spectra (IR), UV-visible diffuse reflectance spectroscopy (DRS), Brunauer–Emmett–Teller (BET) specific surface areas and photoluminescence spectra (PL). The TEM characterization results suggested that the growth of rhombic nanoplates was governed by a nucleation–crystallization–dissolution growth mechanism. The mechanism demonstrated that both the reaction time and the HAc/DMF ratio played crucial roles in controlling size and shape of TiO2 nanocrystals. By tuning the ratio of HAc to DMF, reaction time and reaction temperature, the photocatalytic performance of the as-synthesized TiO2 samples was improved. The enhanced photocatalytic activity could be ascribed to the exposed {010} facets and the high surface areas of the TiO2 nanoplates.

 Please wait while we load your content...
Please wait while we load your content...