Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets†
Abstract
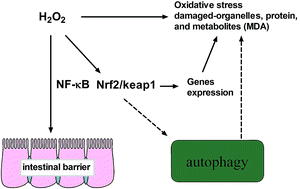
In various pathological conditions of tissue injury, oxidative stress is often associated with autophagy. However, H2O2-induced oxidative stress initiates autophagy and its molecular mechanism is still obscure. Here we report that intragastric and peritoneal administration of H2O2 caused different degrees of oxidative stress and changed intestinal permeability, morphology, and barrier function in piglets. Western blotting studies revealed that H2O2 increased the autophagosome-related protein LC3-I and LC3-II abundance and the ratio of LC3-II to LC3-I content after exposure to 10% H2O2 in the jejunum. Meanwhile, the data from beclin1 also indicated that H2O2 initiated autophagy in response to oxidative stress. In addition, H2O2 activates the NF-κB and Nrf2/Keap1 signals, which may be involved in H2O2-induced autophagy. In conclusion, administration of H2O2 caused intestinal oxidative stress and triggered an autophagic response, which might be associated with NF-κB and Nrf2/Keap1 signals.

 Please wait while we load your content...
Please wait while we load your content...