Novel integrated carbon particle based three dimensional anodes for the electrochemical degradation of reactive dyes†
Rohit Misraab,
Nageswara Nao Neti*a,
Dionysios D. Dionysiouc,
Mahendra Tandekara and
Gajanan S. Kanaded
aWastewater Technology Division, CSIR-National Environmental Engineering Research Institute, Nagpur, 440020, India. E-mail: nn_rao@neeri.res.in; Tel: +91 712 2249885
bInstitute of Water & Wastewater Technology, Durban University of Technology, Durban, South Africa
cDepartment of Biomedical, Chemical and Environmental Engineering (DBCEE), University of Cincinnati, USA
dAnalytical Instrumentation Division, CSIR-National Environmental Engineering Research Institute, Nagpur, 440020, India
First published on 24th December 2014
Abstract
Three-dimensional carbon bed electrochemical reactors have been recently applied for the degradation of several organic pollutants. However, the carbon particles in such reactors slowly undergo attrition. We fabricated a novel flow-through three-dimensional anode using granular activated carbon (GAC) particles and polyvinylidene fluoride (PVDF) binder that potentially avoids such attrition. Optimization of the composition of GAC and PVDF with respect to mechanical integrity and electrical conductivity is reported. The anodes were tested in the electro oxidation of the reactive dyes: Reactive Orange-16 (RO-16), Reactive Red-2 (RR-2), and Reactive Blue-4 (RB-4). A tentative mechanism of dye degradation was proposed based on the observed role of the supporting electrolyte and the cyclic voltammetric, UV-vis, FT-IR and GC-MS data. The decolorization efficiencies were 75 ± 3, 81 ± 5 and 88 ± 4% for RB-4, RO-16 and RR-2, respectively. The integrated 3-D anodes are advantageous because of the absence of carbon attrition, which is otherwise found when a bed of GAC is used in the electrochemical reactors.
1. Introduction
Industries engaged in the manufacture of dyes and dyestuffs, as well as textile dyeing, release treated effluents that retain some residual color. Often, the treated effluents contain non-biodegradable reactive dyes. The discharge of such colored and non-biodegradable effluents continues despite the prevailing regulatory norms in India. These industries are seeking appropriate treatment technologies to target color removal from aqueous effluents. The development of decolorization technologies is also imperative for the textile sector, due to the variability observed in the composition and biodegradability of wastewater.1–5The advanced oxidation processes (AOPs), which are based on the generation of highly oxidizing species such as hydroxyl radicals (HO˙), have demonstrated the capability to mineralize recalcitrant dye compounds.6–8 The treatment of synthetic dye-colored wastewater using AOPs is particularly interesting.3,6–12 One noteworthy approach is photocatalysis using titanium dioxide and sunlight. However, as can be drawn from the previously reported work this method suffers from many setbacks: (i) low oxidation efficiency from real wastewater due to severe matrix ion interference (ii) engineering difficulties in recovering the spent catalyst (iii) low photon-to-OH radical conversion efficiencies (iv) higher capital costs even in pilot scale applications when artificial UV lamps are proposed to be used and (v) large land area requirements for the TiO2/sunlight reactors. In the past decade, the electrochemical oxidation of organic pollutants has attracted wide attention due to the effectiveness and scale up possibilities of the process.13–19 Electrochemical oxidation is also attractive due to its amenability to selectively tune the electrode potential/current to attain higher reaction rates. More importantly, it can also make use of the chloride ions present in many types of common industrial effluents to cause indirect oxidation via the Cl2/OCl-redox couple [8]. Electrochemical oxidation is conveniently carried out in two-dimensional (2-D) parallel plate electrode reactors or three-dimensional carbon bed electrode reactors (TDR).20 TDR have particularly attracted attention due to their high surface-area-to-volume ratios, which result in lower electrical energy consumption, high treatment efficiency, improved mass transfer of pollutants, generation of oxidants in high concentration, and adsorption of pollutants on carbon [8]. Thus, TDR have been successfully used for the treatment of various refractory pollutants such as acid-orange-II,21 formic acid,22 reactive blue 4,8 phenol,23 reactive brilliant red X-3B24 and acid orange-7.25 TDR have also been used to treat actual wastewater, including landfill leachate,26,27 chemical industry wastewater28 and heavy oil refinery wastewater.29 However, the granular activated carbon (GAC) particles forming the carbon bed in TDR are found to undergo slow attrition, which leads to operational problems.27,30
In this study, a novel flow-through three-dimensional (3-D) anode was fabricated using GAC particles and PVDF binder with the aim of avoiding carbon attrition. While the choice of GAC was based on its high surface area and attractive adsorption properties, the choice of PVDF was directed by its tenacious binding properties and thermal stability. PVDF is soluble in N-methylpyrrolidone, which can be washed away with hot water without removing PVDF. The 3-D anode incorporated in a cylindrical stainless steel reactor was applied for the electro oxidation of three reactive dyes; viz., Reactive Orange-16 (RO-16), Reactive Red-2 (RR-2), and Reactive Blue-4 (RB-4). Our results demonstrate that GAC particles can be shaped into an integrated 3D-anode using the PVDF binder and electro oxidation in a three-dimensional flow-through carbon anode reactor (TDFCR), which delivers clean effluent free from the carbon dust that otherwise, arises from carbon attrition. It is pertinent to mention that although PVDF has been frequently used as a binder in the fabrication of carbon based thin super capacitors31 and carbon nanotube–PVDF filter electrodes,32 it has not been employed previously for the preparation of bulk three dimensional integrated particle electrodes similar to the one reported in this study.
2. Results and discussion
2.1 Characterization of granular activated carbon
GAC having 2–3 mm particle size, BET surface area of 600 m2 g−1, iodine content of 500 mg g−1, CCl4 adsorption of 30, and bulk density of 0.60 g mL−1 was used. The surface morphology of the carbon sample is depicted in Fig. 1a. The SEM image confirms a well-defined macro porous structure; moreover, the large pores are tube-like with uniform diameter and possess oval shaped pore openings. The average diameter of the macro pore openings was estimated to be 15–20 μm. Additionally, numerous pin-hole like smaller pores exist in each macro pore. The diameter of these pores is in the range of 0.5–1.0 μm. The nitrogen adsorption–desorption isotherm and pore size distribution plot for the GAC sample are shown in Fig. 1b and c. The isotherm corresponds to a typical porous adsorbent and presents a hysteresis loop at P/P0 between 0.40 and 1.0. This may be caused by a gradual desorption mechanism due to the presence of mesopores. The pore size distribution shows mesoporosity in the range of 3.0–15 nm. | ||
| Fig. 1 (a) SEM images of GAC, (b) nitrogen adsorption–desorption isotherms of typical porous GAC material and (c) pore size distribution plot. | ||
2.2 Electrical resistance and mechanical integrity of the flow through carbon anode
The fabrication of the flow-through carbon anode (TDCA) is described in Section 3.2. The binder composition was varied from 5–15 wt% with respect to the weight of GAC particles, with the objective of attaining a TDCA possessing adequate electrical conductivity as well as mechanical (compressive) strength. The comparison of the % composition, compressive strength and electrical resistance of the fabricated anodes is given in Table 1. The compressive strength was determined using a hydraulic press, while the electrical resistance was determined to establish the electrical continuity of the TDCA in the dry state. Particle–particle contact, which ensures electrical continuity, is critical. At lower binder content (5–8 wt%) the solid ‘integrity’ of the anode was inadequate. The compressive strength was about 5 psi, whereas, at a higher binder concentration (15 wt%), the compressive strength was about 35 psi. However, the anode was ineffective because the binder formed a thick insulating overlayer on the outer surface of the GAC particles. In this study, the inner bulk of the anode did not dry even after a long drying period (>24 h, 100 °C). It is suggested that 10 wt% binder composition is optimum, considering both compressive strength (15.8 psi) and electrical conductance. In this study, all the carbon particles were well integrated and bound to each other tenaciously, leading to an integrated robust 3-D carbon anode. For most purposes, very high compressive strengths are not required. Thus, a self-supporting structure having adequate strength is sufficient to permit handling of the TDCA in electro oxidation reactors.| Composition (%PVDF) | Compressive strength (psi) | Electrical resistance (R = V/I) | Attributes |
|---|---|---|---|
| a NA = not adequate; NM = not measurable. | |||
| 5 | NA | NM | All GAC particles were not held together by the binder and a self-supporting anode could not be obtained as it readily collapsed during removal from the mold |
| 8 | 5.1 | NM | A self-supporting TDCA was obtained, but the TDCA disintegrated with the application of slight pressure |
| 10 | 15.8 | V = 12 V, I = 1 A (R = 12 Ω) | Good conduction was seen and the GAC particles were held together by the binder, resulting in greater mechanical strength |
| 15 | 35.1 | V = 15 V, I = 0.3 A (R = 50 Ω) | Poor conduction was seen but all GAC particles were held together by the binder, resulting in greater mechanical strength. The GAC particles were visibly coated with a whitish PVDF polymer layer, which prevents particle–particle contact and an increase in the electrical resistance. The drying time was also greater |
2.3 Electro oxidation of reactive dyes in the batch mode
A number of batch experiments were conducted using the TDFCR and the three reactive dyes. The applied current (1.0 A) and applied voltage (12.0 V) were fixed for each experiment. Under the experimental conditions, evolution of gases at the electrode was seen, which indicates the rapid occurrence of water electrolysis. The dyes underwent quick decolorization and in a typical batch run 75 ± 3, 81 ± 5 and 88 ± 4% removals were obtained for RB-4, RO-16 and RR-2, respectively. The residual dye concentration in the treated solution was high for RB-4 compared to the other dyes.2.4 Electro oxidation of reactive dyes in continuous mode (TDFCR)
The continuous mode of experiments (HRT-30 min; V = 10 V; I = 1 A) were performed using 50 mg L−1 solutions. UV-vis spectra of the test samples collected at different time intervals during the continuous mode of experiments illustrate the degradation of RR-2, RO-16, and RB-4 (Fig. 2). The residual concentration of the dyes decreased to a minimum and was maintained throughout the experimental duration under electro oxidation conditions for all the reactive dyes. The reactor performed consistently and delivered treated dye solutions having ≤10% residual color compared to the initial color. It was also observed that the effluent from the TDFCR contained very little carbon dust, in contrast to the electrochemical degradation observed with TDR.30RR-2 showed four characteristic bands in its UV-vis spectra (Fig. 2a), one broad band in the visible region (450–600 nm), which corresponds to the conjugated structure connected with the azo group; the other three peaks appear at 331, 285 and 235 nm in the ultraviolet region, resulting from the unsaturated structures of the naphthalene, triazine and benzene rings, respectively. The intensity of all four absorption peaks decreased after electro oxidation, indicating that the azo bond of the dye molecule was ruptured and that the naphthalene, triazine and benzene rings were also transformed during the electro oxidation process in the TDFCR.
The UV-vis spectra of RO-16 recorded for the test samples, before and after electrolysis, (Fig. 2b) show that the electro oxidation process leads to very significant structural modifications. The initial absorption spectrum of RO-16 mainly shows four peaks; 250 and 300 nm in the UV region, and at 390 and 500 nm in the visible region. The absorbance band at ∼500 nm may be due to the n–π* transition of the chromophore azo group (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), and the band at ∼390 nm is due to the π → π* transitions related to the aromatic rings bonded to the azo group. The intensity of the peaks at 500, 390, 300 and 254 nm confirms a rapid decrease in the color concentration due to transformation of the azo group as well as a loss of conjugation in the aromatic groups.
N–), and the band at ∼390 nm is due to the π → π* transitions related to the aromatic rings bonded to the azo group. The intensity of the peaks at 500, 390, 300 and 254 nm confirms a rapid decrease in the color concentration due to transformation of the azo group as well as a loss of conjugation in the aromatic groups.
The initial UV-visible spectra obtained for the RB-4 dye displayed absorption bands at 599, 370 and 296 nm, which is a characteristic of the anthraquinone group, and another band at 256 nm that can be attributed to the aromatic and chlorotriazine groups (Fig. 2c). The final solution after electro oxidation treatment did not show absorption at the wavelengths of maximum absorption, i.e., 599, 370–296, and 256 nm. Both the chromophore (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and the intermediate compounds appeared to have degraded in the reactor.
N–) and the intermediate compounds appeared to have degraded in the reactor.
Additionally, the TDFCR was tested repeatedly at different RB-4 concentrations (50, 100 and 150 mg L−1) by passing the dye solution in single pass mode under applied potential (10.0 V) and current (1.0 A). The UV-visible spectral features are similar for the treated dye solutions obtained over 5 cycles in the continuous mode, with each cycle contributing to one void volume of treated dye solution. The corresponding data given in Fig. 3 suggest that it was possible to maintain a very low residual RB-4 concentration compared to the initial concentration, and the treated water had very faint color even with a higher initial dye concentration. The performance of the TDFCR was tested repeatedly, and a treated volume equivalent to five void volumes (5 × 80 mL) was collected. It can be predicted that the reactor performance could be extended to even more void volumes. Furthermore, RB-4 removal efficiency was 74% in the batch mode compared to 90% under continuous mode. It may be inferred that as the adsorbed dye is electro oxidized at the TDCA, more dye is adsorbed, which further undergoes electro oxidation.
 | ||
| Fig. 3 Percent dye removal versus void volume of the reactor, demonstrating consistent performance with variation in RB-4 concentration. | ||
2.5 Role of the electrolyte
Sodium chloride was added to the dye solution with a view to provide electrolytic conductivity as well as a source of Cl− to induce indirect electro oxidation. In a separate experiment, NaCl was replaced with Na2SO4 to assess the role of chloride ions. NaCl was preferred due to its higher decolorization efficiency. The efficiency of electrolysis depends on the generation of oxidants, with chlorine being the most important oxidant due to the existence of chloride ions. Addition of NaCl can enhance the degradation efficiency and shorten the reaction time, which may be due to the reaction between the generated chlorine/hypochlorite and organic dye molecules. Note that the concentration of NaCl used in the experiments was 2 g L−1. When the NaCl was added, the conductivity of the solution improved, which may also lead to less power consumption.2.6 Cyclic voltammetry of reactive dyes
Cyclic voltammetry is a useful technique which reveals the oxidation–reduction behavior of the original dye molecules and indicates their presence or absence in the reacted dye solutions. This is particularly possible if the dyes are electro-active, as has been found in this study. Moreover, this technique is seldom used for recognizing the presence of dyes in treated solutions, although from our studies it appears to be a very useful tool to quickly identify their presence. The cyclic voltammetric behavior of the dyes in aqueous medium was examined in the potential range of −0.750 to 1.25 V for RO-16, and −0.750 to 0.750 V for both RB-4 and RR-2 (Fig. 4). No well-defined oxidation peaks are seen in the cyclic voltammograms of RO-16 under the specified conditions. A reversible reduction peak was observed at −0.10 V. The reduction current of this peak significantly decreased in the CV corresponding to the treated dye solution. This peak may be attributed to the semiquinone–semihydroquinone transformation. In the case of RR-2, a quasi-reversible reduction peak was found, which may be attributed to the reduction of quinone to the hydroquinone moiety. This reduction peak was nearly absent in the CV corresponding to the treated RR-2 solutions. For RB-4, an oxidation peak was observed at +0.370 V, and a reduction peak with low current occurred at 0.0 V. The observed reduction and oxidation peaks may be attributed to the reversible transformation of the quinone–hydroquinone couple from the RB 4 dye molecule. These characteristic reduction and oxidation peaks were not found in the CV of the electrochemically treated RB-4 solution. The CV data in Fig. 4 confirms that the original dye structures were degraded during treatment in the TDFCR.2.7 By-products of electro oxidation in the TDFCR
GC-MS analysis was carried out on the MTBE extracts of the reacted aqueous dye solutions. The batch electro oxidation experiments were carried out for 4 h with each dye, and also for 20 h in the case of RB 4. The observed compounds, retention time (RT), molecular weight (MW) and m/z values for the major molecular fragments are listed in Table 2. The products (Sr. no. 1–7, Table 2) observed are 4-methylbenzamide, 3-hydroxyl butyric acid, 4-methylamino-butyric acid, substituted nitroso benzylamine, di-butyl phthalate, 1-nitro propanol and N-methylpyrrolidone. Among these, 3-hydroxyl butyric acid, 4-methylamino butyric acid and di-butyl phthalate were observed with all the dyes chosen. N-Methylpyrrolidone (NMP) may be traced to the solvent used in the fabrication of the TDFCA. Several of the by-products are the result of the deep oxidative environment that prevails in the TDFCR. This is also supported by the absence of peaks due to aryl amines (compared with standard azo dye mix 1), which are generally found in dominantly reductive environments (anaerobic degradation).| Name of compound | Structure | MW | RT (min) | m/z | Compound present (✓)/absent (×) in (treated) effluent | ||||
|---|---|---|---|---|---|---|---|---|---|
| RB 4 | RO16 | RR2 | |||||||
| 4 h | 20 h | 4 h | |||||||
| a Post treatment with RO16 (16 h), the reactor was washed with MeOH, dried and reconstituted with 2 mL acetonitrile. | |||||||||
| 1 | 4-Methyl benzamide (toulamide) |  |
135 | 28.52 | 135 (M+); 119 (M+ − 15), 91 (M+ − 44), 78 (M+ − 59), 65 (M+ − 74) | ✓ | × | × | × |
| 2 | 3-Hydroxy butyric acid |  |
104 | 5.14 | 104 (M+), 75 (M+ − 29), 59 (M+ − 45), 47 (M+ − 57), 43 (M+ − 60) | ✓ | — | ✓ | ✓ |
| 3 | 4-(Methylamino) butyric acid | 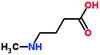 |
117 | 9.45–10.05 | 117 (M+); 100 (M+ − 17); 72 (M+ − 44); 44 (M+ − 73) | ✓ | ✓ | ✓ | ✓ |
| 4 | N-Nitroso-1-phenylmethanamine |  |
136 | 24.96/29.90 | 136 (M+); 105 (M+ − 31), 91 (M+ − 45) | × | — | ✓ | × |
| 5 | Di-butyl phthalate |  |
278 | 33.0 | 278 (M+), 147 (M+ − 131) | ✓ | ✓ | ✓ | ✓ |
| 6 | 1-Nitro-2-propanol | 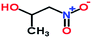 |
105 | 3.35 | 105 (M+); 62, 61 (M+ − 46) | ✓ | × | × | × |
| 7 | 2-Pyrrolidinone, 1-methyl |  |
99 | 9.48 | 99 (M+); 84 (M+ − 15); 70 (M+ − 29); 55 (M+ − 44); 44 (M+ − 55) | × | ✓ | ✓ | ✓ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Reactor washingsa | |||||||||
| 8 | Diazene, bis(1,1-dimethylethyl)- |  |
142 | 12.14 | 142 (M+); 71 (M+ − 71); 57, 41, 29 | ✓ | |||
| 9 | 1-Benzenesulfonylpiperidine-4-carboxylic acid allylamide |  |
308 | 30.93 | 308 (M+); 252 (M+ − 56); 223 (M+ − 85); 167 (M+ − 141); 141 (M+ − 167); 119; 77 (M+ − 172), 55 | ✓ | |||
| 10 | 4-Amino-N-methyl phthalimide |  |
176 | 37.76 | 176 (M+); 132 (M+ − 44), 119 (M+ − 57); 91 (M+ − 85), 77 (M+ − 95) | ✓ | |||
| 11 | 2-Hydroxy-3-methyl-1,4-naphthoquinone |  |
188 | 38.78 | 188 (M+); 160 (M+ − 28); 132 (M+ − 56); 105 (M+ − 83); 77 (M+ − 111); 55 (M+ − 133) | ✓ | |||
| 12 | N,N′-Bis(allyl)-1,4-benzene dicarboxamide |  |
244 | 38.56 | 244 (M+); 188 (M+ − 56), 160 (M+ − 84), 132 (M+ − 110); 104 (M+ − 140), 75 (M+ − 168), 56, 41 | ✓ | |||
| 13 | 1,2,4,5-Tetrazin-3-amine |  |
97 | 4.09 | 97 (M+); 69 (M+ − 28); 55 (M+ − 42); 42 (M+ − 55) | ✓ | |||
Furthermore, after reaction with RO16, the TDFCR was thoroughly washed with water and dried. The compounds that may have remained adsorbed on the TDFCA were extracted by equilibrating the reactor with 120 mL MeOH for 1 h. The MeOH extract was evaporated to dryness under a mild N2 flow. The remaining solid residue was reconstituted in 2 mL acetonitrile and analyzed. The extract contained several degradation products (Sr. no. 8–13 in Table 2), including 4-amino-N-pthalimide, substituted napthadione, and substituted benzamide, which are highly relevant to the structures of the dye compounds used in this study.
2.8 Mechanistic aspects of the degradation of reactive dyes in the TDFCR
The foregoing results clearly confirm that the TDCA is effective for dye degradation. In this study, the reaction efficiency in batch mode was found to be lower compared to the continuous mode of operation. For example, the RB-4 removal efficiency was 74% in batch mode compared with 90% in continuous mode. This may be understood in terms of the favorable mass-transfer phenomenon in the continuous mode of operation, wherein the dye solution moves through the TDCA making contact with all the carbon particles and ensuring the availability of dye molecules at the interface. Thus, the TDCA is more effectively utilized for dye degradation in continuous mode. Furthermore, it was observed that the effluent from the TDFCR contained very little carbon dust. This is in contrast with the observations in our reported study, which concluded that carbon particles in TDR undergo slow erosion and gradually diminish in size with the experimental period.30 Thus, the present TDCA, which is an integrated carbon particle based anode, effectively prevents carbon attrition during the electrochemical degradation of organic pollutants. Though a morphological study is warranted, it can be predicted that PVDF binds the carbon particles, and the resultant three dimensional anode has sufficient electrical conductance to enable the electrochemical degradation of organic dyes.It may be inferred that as the adsorbed dye is electro oxidized at the TDCA, more dye is adsorbed, which undergoes further electro oxidation. Generally, the electrochemical oxidation of organic compounds proceeds through the generation of OH radicals, H2O2, Cl2/OCl−. In the absence of sufficient Cl− concentration, the reaction mainly involves OH radicals produced through water oxidation at the anode, while the complementary reaction at the cathode is usually hydrogen generation. Chen33 reported various aspects of the degradation mechanism during electrochemical oxidation and Rao et al.26 described the role of carbon particles in TDR. In this study, the reaction may be assumed to involve oxidation by the in situ generated OH radicals and chlorine based oxidants.
Based on the results, the various reactions that contribute to the overall electro oxidation of dyes in TDFCR can be written as follows. Reactions (1)–(3) represent water and chloride oxidation at the carbon anode, whereas (4) and (5) illustrate the pH dependent disproportion of electrochemically generated molecular chlorine. The electro oxidation of dye molecules involving hydroxyl radicals and hypochlorite is represented by eqn (6).
2.9 Water and chloride oxidation at the anode
| H2O → ˙OH + H+ + e− | (1) |
| Cl− → Cl˙ + e− | (2) |
| Cl˙ + Cl˙ → Cl2 | (3) |
2.10 Disproportionation of electrochemically generated molecular chlorine & dye oxidation
| Cl2 + H2O → HOCl + H+ + Cl− | (4) |
| HOCl → H+ + OCl− | (5) |
| Dye + ˙OH + (Cl2/H2O) → CO2 + intermediates + inorganic anions + water | (6) |
Though the results suggest that the dye structures are transformed and partially degraded, it was found that some intermediates were formed during electro oxidation in the TDFCR. If these intermediates are present in the treated solutions they can be expected to give rise to absorption peaks particularly in the UV range. However, in the UV-visible spectra of the treated RB-4 solutions it was observed that most spectral features, including those in the UV range, were absent, which implies that all these intermediates either remain adsorbed on the carbon or undergo degradation in the TDFCR. Since carbon is a good adsorbent for many organic compounds, it may be possible that these intermediates remain adsorbed on the carbon. To verify this, we conducted some additional electro oxidation experiments with RB-4 using GAC particulate bed in TDR (6 h electro oxidation reaction of 1000 mg L−1 RB4 solution), and the recovered GAC was processed for recording FT-IR spectrum in ATR mode. The IR spectrum of the recovered carbon was compared with that of carbon deliberately adsorbed with RB-4. The adsorbed RB-4 on carbon showed peaks at 2980–3050, 1743, 1580–1440, 1246, and 979 cm−1. These are attributable to the stretching frequencies of the aromatic –C–H, quinone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, aromatic –C
O, aromatic –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–&–C
C–&–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, –SO3H group and C–Cl bonds, respectively. On the other hand, the recovered carbon prominently displayed stretching vibrations at 2980–3050 due to the aromatic –C–H bond, 1494 cm−1 due to the –C
N–, –SO3H group and C–Cl bonds, respectively. On the other hand, the recovered carbon prominently displayed stretching vibrations at 2980–3050 due to the aromatic –C–H bond, 1494 cm−1 due to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond of chlorotriazine, and 979 cm−1 due to the C–Cl groups of chlorotriazine. The other RB-4 peaks observed at 1743 cm−1 and 1246 cm−1 were clearly absent, indicating that the –SO3H groups were detached and that the anthraquinone mainframe structure was also destroyed during electro oxidation. The main degradation product appears to be chlorotriazine groups as their presence is identified by the peaks at 1494 cm−1 and 979 cm−1. The chlorotriazine groups were also reported as intermediates in similar studies by other researchers.34 Di Giulio et al.34 reported the chromatographic analysis of RB 4 electro oxidation products and observed that the molecule was broken into three fragments i.e., amino anthraquinone sulfonic acid, amino benzene sulfonic acid and the triazinic ring. The triazinic group was reported to resist electrooxidative treatment. On the basis of the GC-MS data of the MTBE extracts of the reacted dye solutions and that of the reactor washings, we may conclude that in this study, intermediates were also formed; moreover, the aqueous phase mainly appears to contain smaller molecular fragments while the larger intermediates are partitioned onto the carbon anode.
N– bond of chlorotriazine, and 979 cm−1 due to the C–Cl groups of chlorotriazine. The other RB-4 peaks observed at 1743 cm−1 and 1246 cm−1 were clearly absent, indicating that the –SO3H groups were detached and that the anthraquinone mainframe structure was also destroyed during electro oxidation. The main degradation product appears to be chlorotriazine groups as their presence is identified by the peaks at 1494 cm−1 and 979 cm−1. The chlorotriazine groups were also reported as intermediates in similar studies by other researchers.34 Di Giulio et al.34 reported the chromatographic analysis of RB 4 electro oxidation products and observed that the molecule was broken into three fragments i.e., amino anthraquinone sulfonic acid, amino benzene sulfonic acid and the triazinic ring. The triazinic group was reported to resist electrooxidative treatment. On the basis of the GC-MS data of the MTBE extracts of the reacted dye solutions and that of the reactor washings, we may conclude that in this study, intermediates were also formed; moreover, the aqueous phase mainly appears to contain smaller molecular fragments while the larger intermediates are partitioned onto the carbon anode.
3. Experimental
3.1 Materials
The reactive dyes, Reactive Blue-4 (RB-4, C.I. no. 61205), Reactive Red-2 (RR-2, C.I. no. 18200), and Reactive Orange-16 (RO-16, C.I. no. 17757) were procured from Sigma-Aldrich (India). The wavelength of maximum absorption (λmax, nm), absorption coefficient (ε, mg−1 l−1 cm−1) and molecular structures of these dyes are given as ESI† (S1). Commercial grade granular activated carbon (INDCARB-30) was purchased from Industrial Carbons Pvt. Ltd., Ankleshwar, India.3.2 Fabrication of the three-dimensional flow-through carbon anode (TDCA)
The carbon anode was fabricated using 100 g of GAC with 40 mL of PVDF binder (10% solution in N-methyl-2-pyrrolidone). A stainless steel pipe (2 mm thickness, 6 cm length and 7 cm ϕ) was used as the electrode casing-cum-cathode. A carbon rod (length, 10 cm; ϕ 0.75 cm) was vertically placed in the center of the SS pipe. The carbon rod served as the anode current collector. The GAC was mixed with the binder, poured into the SS casing and compressed by applying hand pressure. It was dried in an oven at 100 °C for 12 h. The GAC particle anode was then gently pushed out from the SS casing and was washed with hot water until all the excess binder and solvent was removed. The GAC particles were tenaciously bound, and the anode was found to be robust as it also resists moderate mechanical impact. The schematic diagram of a batch electrochemical reactor is given in Fig. 5a. The integrated three-dimensional carbon anode (TDCA) is also illustrated in this figure. The void volume (drainable pore volume representing the volume of reacting dye solution) of the flow-through anode was found to be approximately 80 ± 2 mL.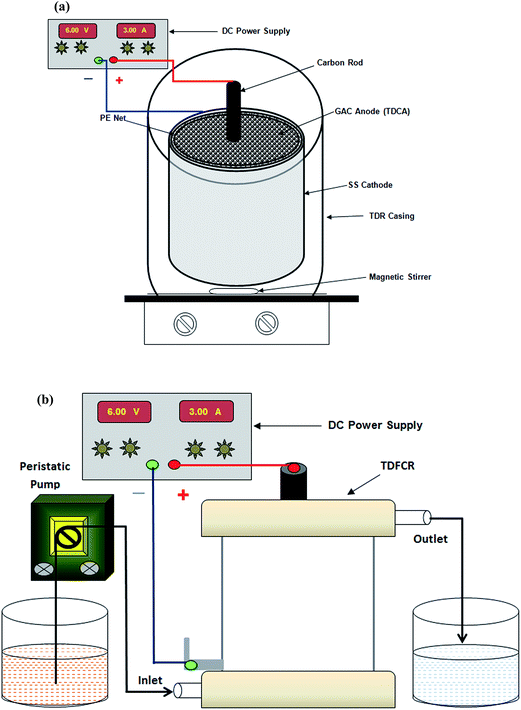 | ||
| Fig. 5 Schematics and experimental set-up of the flow through three-dimensional reactor (TDFCR) in (a) batch mode, and (b) continuous mode. | ||
3.3 Three-dimensional flow-through carbon anode reactor (TDFCR) set-up
The TDCA was wrapped in a polyethylene (PE) net and inserted into the SS pipe. The PE net placed between the GAC anode and the SS pipe cathode acted as an insulating separator to prevent direct contact of the anode and SS cathode, while allowing electrolyte flow. For the batch experiments, the TDCA was directly placed in a glass beaker containing 200 mL of dye solution as illustrated in Fig. 5a. The dye solution was magnetically stirred. Some experiments in continuous mode were performed using the modified flow-through reactor shown in Fig. 5b. Electric power was supplied using a regulated DC source. Wastewater having 50 mg L−1 dye concentration and NaCl (2.0 g L−1) was used as an electrolyte. Salt was added for two reasons: (i) to impart electrical conductivity and (ii) to induce Cl2/Cl−-mediated electro oxidation. In the continuous mode of operation, 1 L of wastewater was fed at a flow rate of 2.7 mL min−1 to maintain a 30 min hydraulic retention time (HRT). In all the experiments, 10 mL reacted samples were carefully withdrawn from the reactor at 30 min intervals and were tested to determine the extent of decolorization. Additional recirculation batch experiments were also conducted using 1.0 g L−1 dye solutions fortified with 4.0 g L−1 NaCl for 4 and 20 h, for the purpose of identifying the by-products of electro oxidation in the TDFCR.3.4 Analyses
The RB-4, RR-2 and RO-16 solutions were analyzed using a Shimadzu UV 1650 PC UV/VIS spectrophotometer at their respective λmax wavelengths (599, 540 and 495 nm). When the run was stopped, the electrolyte was entirely drained from the reactor. The treated electrolyte (effluent) was filtered and analyzed for color using the corresponding UV-vis spectra that were recorded as a function of treatment time. Cyclic voltammetry (CV) were performed for RO-16, RR-2 and RB-4 samples in a standard three-electrode cell comprising Pt working electrode, Pt counter electrode and Ag/AgCl reference electrode using an Autolab PGSTAT 20 instrument.The electrochemical station was equipped with General Purpose Electrochemical System software (Eco Chemie, the Netherlands). The electrode potential was varied from −0.75 to +1.25 V for RO-16, and from −0.75 to +0.75 V for RR-2 and RB-4 at a scan rate of 0.01 V s−1. Sodium sulphate was used as the supporting electrolyte and pure nitrogen gas was used for de-aeration of the test samples for CV studies. A Model JEOL JXA-840A Scanning Electron Microscope (SEM) was used for examining the surface morphology of the carbon. Infrared spectra (ATR) were recorded on a Perkin Elmer Auto Image FTIR spectrometer (Spectrum 100) to ascertain the presence of RB4 and intermediate products on the GAC particles. The electrical resistance of the dry TDCA was measured using an EXCEL DT 9205A digital multimeter. The compressive strength of the TDCA having different weight proportions of PVDF was determined using a hydraulic press (PCI, Mumbai, 0–4000 psi). Nitrogen adsorption and desorption isotherms were measured at −196 °C using a Micromeritics Tristar 3000 gas adsorption analyzer. Before the measurement, the samples were degassed under vacuum (10−4 mmHg at 200 °C) for at least 4 h. The pore size distributions were derived from the adsorption isotherm using the Barrett–Joyner–Halenda (BJH) model.
The samples for GC-MS analysis of the degradation products were processed as follows. Approximately 250 mL of reacted dye solution was placed in a conical flask, and the pH was adjusted to greater than 10 using sodium hydroxide. Then sodium chloride was added until the saturation point was reached. The sample then was extracted twice with 25 mL of methyl-tert-butyl ether (MTBE). The ether fractions were combined; and, moisture was removed over anhydrous sodium sulfate and the fractions were evaporated to dryness. Finally, the extract was reconstituted in 2 mL of methanol for GC/MS analysis. Alternatively, post reaction with RO16 for 16 h, the TDFCR was thoroughly washed with water and dried. The compounds that had possibly remained adsorbed on the TDFCA were extracted by equilibrating the reactor with 120 mL MeOH for 1 h. The MeOH extract was evaporated to dryness under nitrogen gas. The remaining solid residue was reconstituted in 2 mL acetonitrile. The samples were analyzed using GC-MS (Perkin Elmer Clarus 680 GC coupled with 600 C MS, single quadruple MSD) and separation was achieved on an RTX-5 capillary column (30 m length, 0.25 mm ID, 0.25 μm film thickness). The following temperature program was applied: oven temperature, 60 °C (5 min hold time), 60–280 °C@5 °C min−1, final temperature 280 °C (hold for 6 min.); split-less capillary injector temperature, 280 °C; carrier gas: helium@1 mL min−1. The intermediates were identified by comparing with the standard azo dye mix 1 (10 μg mL−1 in acetonitrile) as well as the NIST Library.
4. Conclusions
The electrochemical oxidation of the reactive dyes in TDFCR is efficient with ≥90% color removal. The dyes degrade more efficiently in continuous mode due to the prevailing favorable mass transport phenomenon, which is absent in the batch-mode reactor. The reactor can be used repeatedly for dye degradation, which is mediated by externally supplied Cl− (in the form of in situ generation of the Cl2/Cl− redox couple) and leads to gradual disintegration of the dye molecules into smaller organic fragments. Electro oxidation in the TDFCR delivers clean treated water, free from the carbon dust which otherwise arises from carbon attrition. We conclude that carbon anodes similar to the integrated 3D-carbon anode (TDCA) in this study can effectively prevent carbon attrition during electro oxidation.Acknowledgements
The authors wish to thank Dr S.R. Wate, Director, NEERI for encouragement. The Financial assistance from DST, New Delhi is gratefully acknowledged.References
- Y. Anjaneyulu, N. Sreedhara Chary and D. Samuel Suman Raj, Rev. Environ. Sci. Biotechnol., 2005, 4, 245–273 CrossRef CAS.
- A. B. dos Santos, F. J. Cervantes and J. B. van Lier, Bioresour. Technol., 2007, 98, 2369–2385 CrossRef CAS PubMed.
- F. I. Hai, K. Yamamoto and K. Fukushi, Crit. Rev. Environ. Sci. Technol., 2007, 37, 315–377 CrossRef CAS.
- R. Vinu and G. Madras, Environ. Sci. Technol., 2008, 43, 473–479 CrossRef.
- J. C. Garcia, J. L. Oliveira, A. E. Silva, C. C. Oliveira, J. Nozaki and N. E. de Souza, J. Hazard. Mater., 2007, 147, 105–110 CrossRef CAS PubMed.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1–84 CrossRef CAS.
- H. Kusic, N. Koprivanac, S. Horvat, S. Bakija and A. L. Bozic, Chem. Eng. J., 2009, 155, 144–154 CrossRef CAS PubMed.
- N. R. Neti and R. Misra, Chem. Eng. J., 2012, 184, 23–32 CrossRef CAS PubMed.
- D. Chebli, F. Fourcade, S. Brosillon, S. Nacef and A. Amrane, J. Chem. Technol. Biotechnol., 2010, 85, 555–563 CAS.
- E. Brillas, I. Sirés and M. A. Oturan, Chem. Rev., 2009, 109, 6570–6631 CrossRef CAS PubMed.
- I. Arslan-Alaton, G. Tureli and T. Olmez-Hanci, J. Photochem. Photobiol., A, 2009, 202, 142–153 CrossRef CAS PubMed.
- V. A. Sakkas, M. A. Islam, C. Stalikas and T. A. Albanis, J. Hazard. Mater., 2010, 175, 33–44 CrossRef CAS PubMed.
- N. Anglada, A. Urtiaga and I. Ortiz, J. Chem. Technol. Biotechnol., 2009, 84, 1747–1755 CrossRef.
- J. H. Cho, J. E. Lee and C. S. Ra, J. Hazard. Mater., 2010, 180, 535–541 CrossRef CAS PubMed.
- A. Anglada, A. Urtiaga and I. Ortiz, Environ. Sci. Technol., 2009, 43, 2035–2040 CrossRef CAS.
- N. Mohan, N. Balasubramanian and C. A. Basha, J. Hazard. Mater., 2007, 147, 644–651 CrossRef CAS PubMed.
- R. Boopathy and G. Sekaran, RSC Adv., 2014, 4, 9971–9979 RSC.
- K. Cho, D. Kwon and M. R. Hoffmann, RSC Adv., 2014, 4, 4596–4608 RSC.
- D. Shao, X. Li, H. Xu and W. Yan, RSC Adv., 2014, 4, 21230–21237 RSC.
- X. Li, W. Zhu, C. Wang, L. Zhang, Y. Qian, F. Xue and Y. Wu, Chem. Eng. J., 2013, 232, 495–502 CrossRef CAS PubMed.
- Y. Xiong, P. J. Strunk, H. Xia, X. Zhu and H. T. Karlsson, Water Res., 2001, 35, 4226–4230 CrossRef CAS.
- Y. Xiong, C. He, T. An, X. Zhu and H. T. Karlsson, Water, Air, Soil Pollut., 2003, 144, 67–79 CrossRef CAS.
- Y. Xiong, C. He, H. T. Karlsson and X. Zhu, Chemosphere, 2003, 50, 131–136 CrossRef CAS.
- T. An, X. Zhu and Y. Xiong, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2001, 36, 2069–2082 CrossRef CAS PubMed.
- H.-Z. Zhao, Y. Sun, L.-N. Xu and J.-R. Ni, Chemosphere, 2010, 78, 46–51 CrossRef CAS PubMed.
- N. Nageswara Rao, M. Rohit, G. Nitin, P. N. Parameswaran and J. K. Astik, Chemosphere, 2009, 76, 1206–1212 CrossRef CAS PubMed.
- R. Misra, N. Gedam, S. Waghmare, S. Masid and N. R. Neti, J. Environ. Sci. Eng., 2009, 51, 315–320 CAS.
- S. Masid, S. Waghmare, N. Gedam, R. Misra, R. Dhodapkar, T. Nandy and N. N. Rao, Desalination, 2010, 259, 192–196 CrossRef CAS PubMed.
- L. Wei, S. Guo, G. Yan, C. Chen and X. Jiang, Electrochim. Acta, 2010, 55, 8615–8620 CrossRef CAS PubMed.
- N. Gedam and N. R. Neti, J. Environ. Chem. Eng., 2014, 2, 1527–1532 CrossRef CAS PubMed.
- D. Kalpana, S. H. Cho, S. B. Lee, Y. S. Lee, R. Misra and N. G. Renganathan, J. Power Sources, 2009, 190, 587–591 CrossRef CAS PubMed.
- G. Gao, Q. Zhang and C. D. Vecitis, J. Mater. Chem. A, 2014, 2, 6185–6190 CAS.
- G. Chen, Sep. Purif. Technol., 2004, 38, 11–41 CrossRef CAS PubMed.
- S. Di Giulio, C. Carlesi Jara, D. Fino, G. Saracco, V. Specchia and P. Spinelli, Ind. Eng. Chem. Res., 2007, 46, 6783–6787 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13550d |
| This journal is © The Royal Society of Chemistry 2015 |


