Functional polysaccharides from medicinal mushroom Cordyceps sinensis as a potent food supplement: extraction, characterization and therapeutic potentials – a systematic review
G. M. Shashidhar
ab,
P. Giridhar
c and
B. Manohar
*ab
aAcademy of Scientific and Innovative Research, New Delhi, India
bDepartment of Food Engineering, CSIR-Central Food Technological Research Institute, Mysore, India
cDepartment of Plant Cell Biotechnology, CSIR-Central Food Technological Research Institute, Mysore, India
First published on 19th January 2015
Abstract
As a rich source of novel polysaccharides, Cordyceps sinensis (CS), one of the valued traditional Chinese medicinal fungi, is a major focus of many natural products research efforts. More than 33 polysaccharides have been characterized till the date. Polysaccharides from CS possess a wide spectrum of biological activities like antitumor, antioxidant, immunomodulatory activity, kidney and lung protection, etc. This review covers the recent literature and updates the information on polysaccharides from CS, covering about 130 research articles emphasizing the isolation, characterization of polysaccharides and their biological functions.
1. Introduction
Polysaccharides are biologically active biomolecules composed of simple sugars like glucose, galactose, mannose, fructose, etc. in different ratios showing considerable diversity in their structure and composition. These polysaccharides find applications in food industries as thickeners, binders, stabilizers, emulsifiers, suspending and gelling agents. Production of polysaccharides from microbial sources is preferred which is sustainable and economical as the microbial fermentation utilizes cheap substrates enabling fast and high yield production processes under controlled fermentation conditions.1 Basically polysaccharides are biopolymers, used as a source of therapeutic agents that offer a superlative extent for fetching biological information because of their ability to possess structural variability.2 Shear's polysaccharide which was the earliest polysaccharide found to have an antitumor effect was reported way back in 1943.3 Further numerous polysaccharides have been discovered from mushrooms, fungi, yeasts, algae, lichens and plants. Polysaccharides from fungi enticed world attention for their use due to their potential health benefits such as: antitumor, antioxidative, antiinflammatory, antiviral, immunomodulatory, hypolipidemic and immunestimulatory activities, etc. Bioactive polysaccharides from fungi source with unbelievable clinical applications have been found in traditional Chinese medicines (TCM). Since these molecules are potent to demonstrate wide spectrum of biological properties, they are suitable for their application in many quite idiosyncratic areas, such as food, nutraceutical, biomedicine and cosmetic industries. Generally these polysaccharides remained non-toxic, possesses clinical efficacy in controlled trials while the majority of such compounds remain as nutraceuticals in preliminary research.4 Most of the documented immunoactive polysaccharides from medicinal fungi are β-glucans and α-(1,3)-D-glucans wherein latter are less common than former but have been found in the cell walls of most respiratory pathogenic fungi5 and linear α-(1,3)-D-glucans extracted from the fruiting bodies of Agrocybe cylindracea and Amanita muscaria had significant immunomodulatory activities after carboxymethylation.6,7 Polysaccharides from other fungi sources are: Lentinan from Lentinula edodes (Berk.) Pegler,8 Schizophyllan from Schizophyllum commune Fr.,9 more commonly protein bound polysaccharide K (PSK) from Coriolus versicolo (L.) Quél.10,11 which have been found to aggrandize and stimulate the immune system.12 Market size for food polysaccharides as additives is over $US 3 billion which includes major polysaccharides like starch and gelatine accounting for about 50%.13Cordyceps sinensis (CS) is a one of the valuable traditional medicinal fungi among Chinese medicines having long history. Over the millennia, CS has been treasured throughout Asia as one of the most effective natural tonics to strengthen vitality and promote longevity. Since CS possesses number of far reaching health effects, it is regarded as one of the cornerstone of traditional Chinese medicines for centuries.14 CS is basically entomophagous fungus, parasitizes larvae of ghost moths (Hepialus armoricanus) and produces a fruiting body assessed as an herbal remedy. It is mainly distributed in China, Tibetan Plateau, Bhutan, Nepal and northern part of India at an altitude of 3500–5000 metres above sea level. In Chinese, it is also called as Dong Chong Xia Cao which means “winter worm summer grass”15,16 and often known as a Himalayan Viagra. In Chinese Pharmacopeia, CS has been regarded as a celebrated drug since 1963, it is found to have similar medical effects of ginseng and deer velvet.17 CS contains major class of active ingredients like nucleosides, polysaccharides, sterols, etc. and products formulated from these have gained great popularity in Eastern medicines. In view of the above facts, there is an increased worldwide demand for CS which has led to overharvesting and subsequent meagreness of wild species. Hence researchers hunted for alternative approaches to produce CS artificially by bioreactor cultivating technology to meet human needs and to mitigate the pressure on natural resources of the species.
In previous review article,18 potent bioactive principles from CS, extraction methods, health benefits and other aspects have been discussed in detail. Since polysaccharides from CS are also believed to be major contributors to overall pharmacological effects, a systematic, informative review is felt necessary. This review will conceptually illustrate research activities carried out till date on submerged fermentation of CS for polysaccharides production and to promote CS as health foods, functional foods or nutraceuticals. The methods, technologies and instruments used to isolate, purify and elucidate polysaccharides structures are also covered.
2. Cultural conditions for optimum polysaccharides production
Initial attempts to develop an efficient technology for cultivation of fruiting bodies became futile because of major limitations.19 Submerged fermentation is a popular alternative method for large scale cultivation of CS to meet the world demand. Nutritional composition of media, temperature, pH and other supplements play decisive role in production of biomass and strongly affect the chemical composition, structure and productivity of polysaccharides. The biomass and the yield of polysaccharides in the biomass will be low in the basal medium and so optimisation studies are necessary. These optimal cultural conditions vary among CS strains. Fig. 1 lists optimum cultural conditions for the maximum production of both biomass and polysaccharides. Kim and Yun (2005) have studied the effect of carbon, nitrogen sources and minerals on highest biomass and polysaccharides in CS. The study suggests the optimal cultural conditions and also suggests using different sugars, organic nitrogen sources like corn steep powder and Ca+, Mg+ minerals combination results in better biomass yield (20.9 g L−1) and polysaccharides (4.1 g L−1) production.20 A study by Hsieh et al. (2005), demonstrates the effect of different carbon sources (fructose, glucose, sucrose, mannose, maltose and molasses), percentage of nitrogen source (0.25–1.5%), and pH of the medium. Regression model based on response surface methodology (RSM) predicted the optimum parameters like sucrose (6.17%), corn steep powder (0.53%) and pH 4.4 (pH lower than 3 favours) for maximum polysaccharides yield of 3.17 g L−1.21 Another study suggests mass production of mycelium and exopolysaccharides from CS 16 is possible with the modified basal media (PDB) composition (2% sucrose, 0.9% yeast extract, 0.3% K2HPO4, and 0.4% CaCl2).22 Some studies intended to maximize polysaccharides content via media optimization.23–25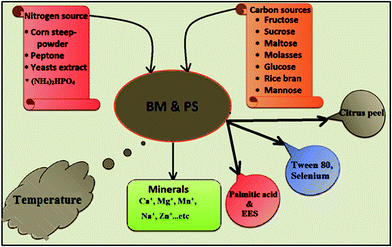 | ||
| Fig. 1 Significant impacts of various cultural conditions and additives on biomass and polysaccharides production. BM-biomass; PS-polysaccharides; EES-ether extract of Eupolyphaga sinensis. | ||
Few studies demonstrated the use of compounds other than sugars, nitrogen sources and minerals that have significant impact on biomass and polysaccharides production. Palmitic acid is basically a fatty acid and ether extract of Eupolyphaga sinensis have shown to act as stimulator of cell growth and extracellular polysaccharides of CS. Addition of above mentioned stimulators along with the modified basal media showed improved biomass and polysaccharides production by 1.5 fold.26 Liu and Wu (2012) investigated the effects of surfactant additives and medium pH on mycelia morphology and EPS production in liquid culture of a valuable medicinal fungus CS-HK1. Tween 80 (polysorbate 80) is one of the most favourable surfactants for EPS production by many microorganisms including medicinal fungi and found to enhance growth and metabolite production. The mechanism behind these effects is due to its surface active properties, lowering the mycelium liquid interfacial tension and thus the potential or tendency of mycelia to form aggregates. A decrease in the surface tension of the medium by a surfactant lowers the thermodynamic potential for the aggregation but favours the dispersion of mycelia.27 Selenium (Se) is an essential trace element of glutathione peroxidase (GSH-Px) that participates in synthesis of enzymes and protects the structure and function of biomembrane from over oxidation and damage. Researchers concluded that addition of Se to medium can potentially enhance the antioxidant activity of polysaccharides which in turn enhances adaptive immune responses.28 The study also revealed that the addition of citrus peel which is a source of pectin and flavonoids, along with culture broth containing different carbon sources, principally rice bran could enhance polysaccharides content and their anticomplementary, radical scavenging activities.29 Supplementing liquid medium with ammonium had stimulated the production of EPS in mycelial culture of CS. The ammonium feeding has increased the EPS production by 40% and also had a slightly positive effect at 5–10 mM L−1, but a negative effect at higher concentrations on the mycelium growth.30
Solid fermentation method of producing CS mycelia is not successfully followed because of constraints in recovering biomass free of contamination. Polysaccharides were extracted from CS mycelium grown on solid media containing soybean meal and rice bran (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) where the optimum inoculation amount, fermentation temperature, water content of medium, air relative humidity and fermentation time were found to be 20%, 26 °C, 60%, 60% and 7 days, respectively.31
2 w/w) where the optimum inoculation amount, fermentation temperature, water content of medium, air relative humidity and fermentation time were found to be 20%, 26 °C, 60%, 60% and 7 days, respectively.31
3. Extraction of crude polysaccharides
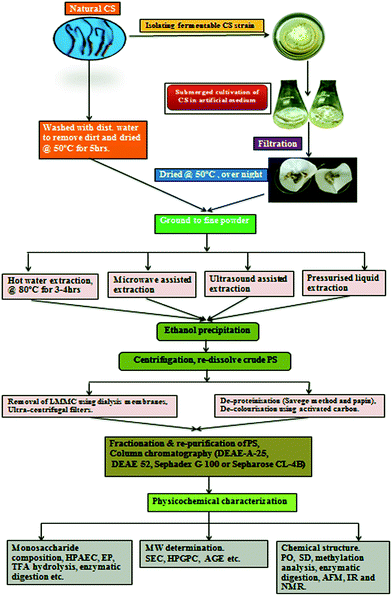
Wild whole fungus (both fruiting body and dead caterpillar) and mycelium of CS are the two main sources of polysaccharides and many studies have been reported on the extraction of polysaccharides from CS. Extraction of polysaccharides from CS and their far reaching bioactivities has become a research interest since polysaccharides have gained potential commercial importance.32 Polysaccharides are basic components of cell wall of fungal cell that are arranged to create three dimensional network, literally saying they are armour of cell wall, which is dynamic as a result of modifications in cultural conditions and environmental stresses. Basically cell wall is an insoluble structure composed of two major types of polysaccharides one is a rigid fibrillar of chitin (or cellulose), and the other is a matrix like β-glucan, α-glucan and glycoproteins33 that must be solubilized to be precisely analyzed.34 Appropriate downstream extraction techniques are required to obtain above class of compounds. Basically polysaccharides are polar in nature, extraction with polar solvents like hot water, hot alkali solution, etc. enables isolation and purification of the polysaccharides according to their different solubility in water and organic solvents, or based on their different ionic properties and molecular weight distributions.35 The intention to extract polysaccharides at high water temperature or probably at ebullition temperature and from mild to strong pH condition is to break the cell wall from outer layer to the inner layer. Extracted polysaccharides can be further purified using a combination of techniques, such as ethanol precipitation, fractional precipitation, acidic precipitation with acetic acid, ion exchange chromatography, gel filtration, and affinity chromatography.35 Miyazaki et al. (1977) for the first time obtained water soluble crude polysaccharides from ascocarps of CS by hot water extraction and ethanol precipitation.36However, the major drawbacks of hot water extraction are the high extraction temperature, long extraction time and low extraction efficiency. Various methods have been used to improve the extraction efficiency such as treatment with enzymes like cellulose,37 microwave,38 high pressure homogenization and high power ultrasound.39 Fig. 2 depicts steps involved and methods, techniques followed to extract, purify and characterize the polysaccharides from CS.
4. Purification and characterization of polysaccharides
The crude polysaccharides contain low molecular mass compounds and proteins, so few steps are required to be followed to purify and elucidate its structural features. Table 1 shows the structural features of polysaccharides reported in literature.| POLYS | Monomers compositional ratio | Main chain | Branch | MW (kDa) | Bioactivities | Reference |
|---|---|---|---|---|---|---|
| a POLY-polysaccharides; S-source of CS; C-cultured; N-natural; FCC-fruiting bodies of cultured CS; UNK-unknown. | ||||||
| APSFC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 3.5 Gal = 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
— | — | — | Immunomodulatory effects | 89 |
| EPS-1AC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga l = 15.2 Ga l = 15.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
(1 → 6)-α-D-Glucose residues (∼77%) and (1 → 6)-α-D-mannose residues (∼23%) | (1-6)-α-D-Mannose residues and (1 → 6)-α-D-glucose residues at O-3 position of (1 → 6)-α-D-mannose residues of the backbone | 40 | — | 41 |
| AEPSC | Glcp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GlcUp = 8 GlcUp = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a trace amount of mannose 1 with a trace amount of mannose |
(1 → 3)-linked α-D-Glcp | α-D-Glcp and α-D-Glc up, attached to the main chain by (1 → 6) glycosidic bonds at every 7th α-D-Glcp unit | 36 | Immunomodulatory effects | 7 |
| PS-AC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Gal D-Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Man = 2 D-Man = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
→3-α-D-Glcp-1 → 3-β-D-Glcp-1 → 3-β-D-Galp-1→ | Branch residue (α-D-Manp–1→) linked at the O-2 position of residue 3-α-D-Glcp-1 | 460 | Antihypercholesterolemia | 51 |
| WIPSC | α-D-Glucose | (1 → 4)-linked α-D-Glcp | (1 → 6)-linked α-D-Glcp | 1180 | Antitumour & immunostimulating effects | 104 |
| AIPSC | α-D-Glucose | (1 → 4)-linked α-D-Glcp | — | 1150 | Antitumour & immunostimulating effects | 104 |
| CBHPC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 95.19% Gal = 95.19%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.91% 0.91%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.61% 0.61% |
Glcp joined by 1 → 4 linkages and 1 → 3 linkages | The branching points are located at O-2 or O-6 of Glcp with α terminal-D-Glcp as side chain | — | Antifibrotic effect | 49 and 72 |
| CS-PpC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 21 Gal = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1,3-β-D-Glucan | 1,6-branched chain | — | Monocyte activation | 90 |
| CPS1C | Glu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara = 46 Ara = 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1,6-Man | Glucose at C2, C3, C4 position | 99.1 | Kidney protection | 71 |
| CPS2C | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GlcUA GlcUA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ara Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rha = 30 Rha = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 : 3 4 : 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1,6-Glc, 1,6-Man, 1,6-Gal | Different monosaccharide residues at C3 position | 25.6 | Kidney protection | 71 |
| PCB IIC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1 Glc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.51 0.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
— | — | ∼60 | Immunoenhancing & tumorinhibiting effects | 128 |
| PCB IC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 1 Gal = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.73 0.73 |
(1 → 4)-linked Manp | Galf and Manp | ∼60 | Immunoenhancing & tumorinhibiting effects | 128 |
| PCA IC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 1 Gal = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(1 → 4)-linked Manp | 1 → 2, 1 → 3, 1 → 6 linkages Galf and Manp | ∼556 | Immunoenhancing & tumorinhibiting effects | 128 |
| PC IC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1 Glc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.65 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.30 0.30 |
— | — | ∼350 | Immunoenhancing & tumorinhibiting effects | 128 |
| Glucomanno-galactanFCC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 2.8 Gal = 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Long backbone of (1 → 2), (1 → 4)-linked Man units and (1 → 3,6)-Glc units | (1 → 3)-linked Gal units and (1 → )-linked Glc units | 8.1 | Antioxidant activity | 44 |
| CAPSC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc |
— | — | 2.7 | Kidney protection | 81 |
| PolyhexNAcC | — | -4-β-D-ManNAc-(1 → 3)-β-D-GalNAc-(1→ | -Gal- at 3-position of ManNAc | 6 | Antioxidant activity | 128 |
| UNKC & N | Glc and Gal | (1 → 4) linked-α-D-Glc units, (1 → 4) linked-β-D-Glc units and (1 → 4)-linked α-D-Gal units | — | — | — | 43 |
| UNKN | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 1.00 Gal = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.61–3.82 16.61–3.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.60–1.28 1.60–1.28 |
— | — | — | — | 50 |
| CS-81002C | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 10.3 Glc = 10.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
→6-)-Man-(1→ | →3,6-)-Man-(1→side chains at C3 position→2,6-)-Man-(1→side chain at C2 position | 43 | Immunostimulating effect | 52 |
| CHWpC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Gal D-Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Glc = 1.0 D-Glc = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8 |
— | — | ∼32 | Hypoglycaemic activity | 133 |
| MannoglucanC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc = 1 Glc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
(1 → 4)-and (1 → 3)-linked α-D-glucan | α-D-(1 → 6)-Manp | 7.7 | Anticancer effect | 130 |
| PSC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Man D-Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-Ara L-Ara![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Gal = 8 D-Gal = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | ∼83 | Immunomodulatory effect | 91 |
| CordyglucansC | Glucose | (1 → 3)-linked backbone | (1 → 6)-linked branches | 12.86 | Antitumor activity | 105 |
| D-GlucanC | Glucose | (1 → 3)-β-D-Glucosyl residues | (1 → 4)-β-linked D-glucosyl residue | 13.62 | Antitumor activity | 105 |
| SCP-IC | Glucose | α-(1 → 4)-linked backbone | α-(1 → 6)-linkage | 184 | — | 54 |
| CS-F10C | Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = 43 Man = 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
(1 → 5 and/or 6)-linked β-D-Galf residues | (1 → 2)-linked α-D-Manp residues | 15 | Hypoglycemic activity | 111 |
| CS-F30C | Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glc Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man = 62 Man = 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
— | — | 45 | Hypoglycemic activity | 109 and 110 |
| CSP-1C | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 1 Gal = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
— | — | ∼210 | Antioxidant activity; hypoglycemic activity | 48 |
| PSCSN | — | — | — | 100 | Antitumor effect | 96 |
| CT-4NN | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Gal = 3 D-Gal = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
(1 → 6)-and (1 → 2)-linked α-D-Manp residues | (1 → 5)-linked β-D-galf residues, and (1 → 6)- linked α-D-galp residues | ∼23 | — | 131 |
| CS-IN | Gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-Man = 1 D-Man = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(1 → 2)-α linked-D-Manp residues | (1 → 3), (1 → 5), and (1 → 6)-linked-D-galf, (1 → 4)-linked D-galp residues | — | — | 36 |
| EPSC | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Glu Glu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 23 Gal = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
— | — | 104 | Immunomodulatory & antitumour | 82 |
| CordysinocanC | Glc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Man Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 2.4 Gal = 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 82 | Immunomodulatory activity | 83 |
| HS002-IIC | — | Long backbone of (1 → 3)-linked α-D-ribofuranosyl units, (1 → 4)-linked α-D-xylopyranosyl units and (1 → 4)-linked β-D-glucopyranosyl units, substituted at C-6 position | β-D-Mannopyranosyl residues and β-D-galactopyranosyl residues terminated with α-L-arabinopyranosyl residues | 44 | Immunomodulatory activity | 88 |
| CS-PSC | Man, Rha, Ara, Xyl, Glc, and Gal | — | — | 12 | Antioxidation activity & modulate immune function | 97 |
| CME-1C | Man![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gal = 4 Gal = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
Backbone of (1 → 4)-linked mannose | (1 → 6)-linked galactose residues attached to the O-6 of mannose | 27.6 | Cytoprotective effect | 127 |
4.1 Removal of low molecular mass compounds
Low molecular mass compounds associated with crude polysaccharides are removed by means of dialysis through a 12–14 kDa membrane,40,41 ultracentrifugal filters with molecular mass cutoff: 10 kDa,42 3 kDa.434.2 Deproteinisation
Protein fraction was separated from crude polysaccharides by Sevage method.40,44 Along with Sevage method, papain enzyme was also used to deproteinise the crude EPS and further decolorized by treating with activated carbon.414.3 Fractionation
Deproteinised crude polysaccharides are subjected to fractionation to elute different fractions. Acidic polysaccharide was isolated from the EPS fraction by ion exchange chromatography (IEC) on DEAE cellulose-52 and further isolated acidic polysaccharide was purified by gel permeation chromatography (GPC) on a Sephadex G-75 column.45 Wang et al. (2011) used KTA Explorer chromatography system to fractionate crude EPS and eluted fractions were further repurified by running Superdex 200 HR 10/30 column.264.4 Physicochemical characterization
The physicochemical and structural features of a polysaccharide include monosaccharide composition, molecular weight, configuration of glycosidic linkages, type of glycosidic linkage, position of glycosidic linkage, sequence of monosaccharide, number and location of appended non-carbohydrate groups, and molecular chain conformation.46,47Analysis of monosaccharide composition analysis involves cleavage of glycosidic linkages by acid hydrolysis, derivatization, detection and quantification by GC. In addition, high performance anion exchange chromatography with pulsed amperometric detection has been gradually developed to supplement traditional methods as it does not require derivatization of monosaccharide with high resolution. Fig. 3 shows the monosaccharide composition of different polysaccharides extracted from natural or cultured CS. Li et al. (2003) analysed CSP-1 for composition by capillary electrophoresis by using TFA and found that CSP-1 contained glucose, mannose and galactose in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75.48 Monosaccharide composition of CBHP was determined by acid hydrolysing and analysed by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The results revealed that CBHP consisted glucose, mannose and galactose (95.19%, 0.91% and 0.61%, respectively).49 Polysaccharides extracted by pressurized liquid method were analysed for their sugars composition by TFA hydrolysis, derivatization by treating hydroxylamine hydrochloridepyridine solution and GC-MS. The polysaccharides were found with mannose, glucose and galactose residues with a molar ratio of 1.00
0.75.48 Monosaccharide composition of CBHP was determined by acid hydrolysing and analysed by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The results revealed that CBHP consisted glucose, mannose and galactose (95.19%, 0.91% and 0.61%, respectively).49 Polysaccharides extracted by pressurized liquid method were analysed for their sugars composition by TFA hydrolysis, derivatization by treating hydroxylamine hydrochloridepyridine solution and GC-MS. The polysaccharides were found with mannose, glucose and galactose residues with a molar ratio of 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.61–3.82
16.61–3.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.60–1.28.50 A heteropolysaccharide, PS-A was subjected to activity guided fractionation and composition was analysed. PS-A composed of D-glucose, D-galactose, and D-mannose at a molar ratio of 2
1.60–1.28.50 A heteropolysaccharide, PS-A was subjected to activity guided fractionation and composition was analysed. PS-A composed of D-glucose, D-galactose, and D-mannose at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.51 Composition of CS-81002 was determined after acid hydrolysis by methylation analysis and results showed that it composed of mannose, galactose and glucose in the ratio of 10.3, 3.6 and 1.52
1.51 Composition of CS-81002 was determined after acid hydrolysis by methylation analysis and results showed that it composed of mannose, galactose and glucose in the ratio of 10.3, 3.6 and 1.52
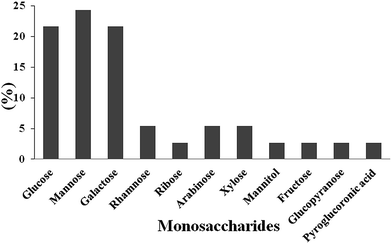 | ||
| Fig. 3 Monosaccharide compositions (MC) of different extracted polysaccharides from natural or cultured Cordyceps sinensis. Data referred from Soltani et al. (2013).132 | ||
Molecular weight is one of the essential physical characters of a polymer and various techniques such as viscometry, osmometry, sedimentation, and HPLC have been used to determine the average polymer MW and polydispersity index. Also high performance gel permeation chromatography (HPGPC), and size exclusion chromatography with multi angle laser light scatter detection are an efficient methods for the evaluation of the absolute MW of polysaccharides which provides greater resolution than traditional gel permeation chromatography.53 Leung et al. (2009) characterized the EPS for their MW by using GPC, comparing against dextran MW standards. GPC spectrum showed five peaks correspond to molecules with MW range from about 5 kDa to more than 200 kDa.40 High performance size exclusion chromatography (HPSEC) analysis was also followed to determine MW of water soluble polysaccharides from CS.42 Combination of HPGPC and agarose gel electrophoresis techniques have been used to determine the MW of AEPS-1, an acidic polysaccharide fraction.45
Chemical structural features of polysaccharides can be derived by using techniques such as Periodate oxidation, Smith degradation, methylation analysis, enzymatic digestion, AFM, IR and NMR analyses. Miyazaki et al. (1977) derived the chemical structure of CS-1 by Periodate oxidation, Smith degradation, methylation analysis, partial hydrolysis and 13C-NMR spectrometry. The data showed CS-1 consists of mannan core and galactosyl oligomer as a branch chain. The mannan core mainly contains α-(1 → 2)-linked mannopyranosyl residues and branch chain consists of (1 → 3), (1 → 5) and (1 → 6) linked D-galactofuranosyl and (1 → 4)-D-galactopyranosyl residues.36 EPS-1A is a novel polysaccharide from CS characterised using FTIR and NMR techniques.41 Guan et al. (2011) compared and characterized polysaccharides from natural and cultured CS by enzymatic digestion method where cellulase, lichenase, β-(1,4)-D-galactanase, β-mannanase, α-amylase, and isoamylase have been selected for the study.19 Few studies have been reported in literature aimed at elucidating the polysaccharides structures from CS43,49,54 and has been listed in Table 2.
| Polysaccharides | Structure | Method used | Bioactivity | Reference |
|---|---|---|---|---|
| a CS-correlation spectroscopy; NOS-nuclear overhauser spectroscopy; SD-Smith degradation; PO-Periodate oxidation, AFM-atomic force microscopy; AGE-agarose gel electrophoresis; HPGPC-high performance gel permeation chromatography; GF-gel filteration; DOSY-diffusion ordered spectroscopy. | ||||
| CS-81002 | 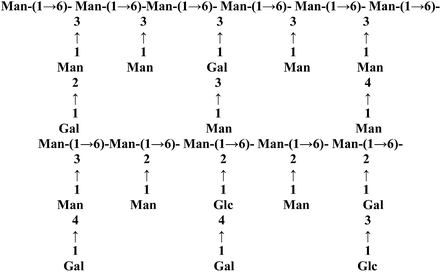 |
GF, methylation, GC | Immunostimulating effect | 52 |
| HS002-II | 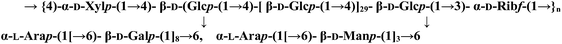 |
FTIR, HPGPC, AGE, AFM, NMR | Immunomodulatory activity | 88 |
| CBHP | 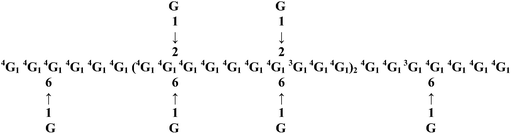 |
Methylation analysis, 1D and 2D NMR spectroscopy | Antifibrotic effect | 47 and 72 |
| SCP-I | 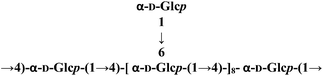 |
Methylation, SD degradation, acetolysis, NMR | — | 54 |
| EPS-1A |  |
GC, GC-MS, FTIR, 1H NMR and 13C NMR, acid hydrolysis, methylation, PO and SD | — | 104 |
| PolyhexNAc | 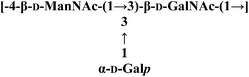 |
MS, methylation and NMR | Antioxidant activity | 129 |
| Mannoglucan |  |
FTIR and NMR | Anticancer effect | 130 |
| PS-A |  |
1H, 13C NMR, CS, and NOS | Anti-hypercholesterolemia | 51 |
| CME-1 | 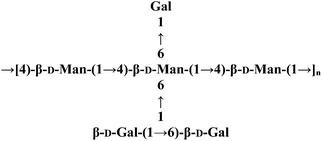 |
GF, GC-MS and NMR (DOSY) | Cytoprotective effect | 123 |
5. Bioactivities of polysaccharides
Both dead larva and fruiting body of CS have been used as traditional medicine and health food for hundreds of years for “lung invigoration and kidney nourishment” in China and moreover it has been classically characterized as a tonic in nature. Many studies in vitro and in vivo support that bioactive polysaccharides from CS have diverse biological activities and pharmacological potential supporting human health.5.1 Liver protection
CS has been used to treat liver disease in traditional Chinese medicine for thousands of years. Polysaccharide extracted from CS is the major active components with antiliver injury effects. A study evaluated the protective effects of six kinds of polysaccharides from different origins of Cordyceps on immunological liver injury in mice. Polysaccharides were given at ig 150 mg per day for 12 days and the activities of serum transaminase (ALT, AST) and liver SOD, the concentration of liver MDA, the weighting indexes of liver and spleen, the liver pathology was comparatively observed. The result reflects the armouring effects of polysaccharides on immunological liver injury in mice wherein decreased level of MDA and increased activity of SOD in the liver homogenates (P < 0.05–0.01) were observed.55 Similar effects were also observed in a couple of studies.56–58 Polysaccharides were found to prevent liver fibrosis and improve the function of liver and peripheral blood lymphocyte in patients with chronic hepatitis C.59 In one of the study, CS polysaccharides were proven to have antifibrotic effect with their potent application in liver fibrosis. Polysaccharides dose at 10 μg mL−1, type I and type III procollagen mRNA levels were inhibited at the rate 27.9% and 45.4% (P = 0.05 and P = 0.05, respectively) thus concluding these can markedly inhibit cell proliferation and collagen synthesis in a dose and time dependent manner, and down regulate the expression of procollagen type I, III mRNA in vitro.60 Another similar study also revealed inhibitory action of CS polysaccharides on Hepatic stellate cells (HSC) proliferation and collagen synthesis.61Peng et al. (2013) investigated the antiliver injury effect of polysaccharides against carbon tetra chloride (CCl4) where colchicine was used as a positive control. They observed the effects on hepatic stellate cell (HSC) activation, transforming growth factor-β1 (TGF-β1)/Smad pathway, as well as matrix metalloproteinase MMP2, MMP9 and tissue inhibitor of metalloproteinase TIMP1, TIMP2 and inhibition of liver injury and fibrosis was confirmed by drop in serum alanine aminotransferase, aspartate aminotransferase, total bilirubin, hepatic hydroxyproline and rise in serum albumin, as well as alleviation of histological changes.62 In a similar study pharmacological effect of Cordyceps polysaccharide on dimethylnitrosamine (DMN) induced liver fibrosis in rats was investigated63 and the treatment of polysaccharides showed significant reduction of protein expression of proliferating cell nuclear antigen (PCNA) in liver tissues.64 Cordyceps polysaccharides had also a significant suppression effect on hepatic stellate cells of rat, and the activity of nuclear factor κB, and down regulated the expression of cytokine tumor necrosis factor in a dose dependent manner. It also reduced the proliferation of hepatic stellate cells by inhibiting the activity of NF-κB and the expression of TNF-α and also lowering the level of protein.65
Fang et al. (2000) discovered the mechanism behind the action of polysaccharides on liver fibrosis induced by immunologic injury in rats. Results concluded that Cordyceps polysaccharides attenuate liver fibrosis, decrease hepatic Hyp content and collagen production, reduce transforming growth factor β1 and its receptor expression and decrease cell Dm expression.66
5.2 Kidney
According to traditional Chinese medicines, the kidneys are known as “root of life”, CS goes to kidney meridian, provides kidney improvement and has been reported kidney nourishment.15,67–69 A water soluble polysaccharide (CPS-2) isolated from CS probably composed of glucose and mannose residues having molecular weight of 43.9 kDa was found to possess protective effect on the model of chronic renal failure. The effect was confirmed as changes in blood urea nitrogen and serum creatinine and revealed that CPS-2 could significantly relieve renal failure caused by fulgerizing kidney.70 In similar study two polysaccharide fractions named as CPA-1 and CPS-2 separated by alcohol and DEAE-cellulose showed to provide protection effect to the kidney cells and this study provide scientific basis for the beneficial effect of soluble polysaccharides in renal disease.71In ureteric obstruction (UUO) model of renal fibrosis and tubular epithelial cell line HK-2, histological and immunohistochemical assessment of renal injury was made at day 7 and treated with characterized fraction of polysaccharide (Cp-F1) of CS.72 The significant attenuation of UUO was noticed, evidencing the antagonist activity by CS, mediated via large soluble polysaccharide aggregate, preventing induction of fibronectin and α-SMA, and inhibition of the epithelial cell marker E-cadherin. Furthermore Cp-F1 inhibits TGF-β1 dependent activation of a Smad signalling and suppresses the expression of TGF-β receptor mRNA and protein.72 In the similar studies, acute kidney injury in rat was induced by denaturation haemoglobin and treatment with Cordyceps polysaccharides showed significant palliation in injury of renal function (P 0.01) and renal pathologic changes (P 0.01) concluding its possible mechanism maybe related to improve renal function, rectify abnormal metabolism, promote renal tubular restoring and regeneration.73 The therapeutic effects of Cordyceps polysaccharide on occluding renal artery induced acute kidney injury in dog and on adenine induced chronic renal failure (CRF) in rats. Renal function, biochemical indicators, kidney index, renal pathologic changes were observed after treatment confirming the protective effect of polysaccharides on kidney and kidney improvement.74,75
An investigation discovers therapeutic effect of Cordyceps polysaccharides on the kidneys frozen chronic renal failure in rats is available. The study was conducted on rats divided into six groups among which three groups were treated with high, medium and low dose of polysaccharides 160, 80 and 40 mg kg−1, respectively. After administration biochemical indices of blood of the rats, serum total superoxide dismutase and malondialdehyde (MDA) levels were detected as well as changes in renal pathology. Results concluded that Cordyceps polysaccharides can be effective in preventing the occurrence of chronic renal failure and development, improve renal function, correct metabolic disorders, and promote the repair and regeneration of renal units.76 Chen et al. (2009) established CRF rat model by 5/6 nephrectomy to study the therapeutic effects of polysaccharides and biochemical indicator, renal function, and renal pathologic changes were observed confirming the renal function improvement.77 Few studies brief the protective effects of polysaccharides on renal injury.78–81
5.3 Immunomodulatory effects
In recent years, studies explored the potential modulating effects of polysaccharides from CS, on stimulation or suppression components of the immune system. Polysaccharides were shown to be immunomodulators, effective against treating or preventing diseases and illnesses that stem from certain immunodeficiencies and other depressed states of immunity.Exopolysaccharides (EPS) from CS were found to possess both immunomodulatory and antitumor effects by activating the immunocytes and promote cytokines expressions. Splenocytes were treated with EPS having molecular weight about 1.04 × 105 at different doses and different treatment timings. EPS elevated proliferation ability of spleen lymphocytes only at 100 μg mL−1 after 48 h treatment and tumor necrosis factor alpha (TNF-α), interferon-α (IFN-γ), and interleukin-2 (IL-2) mRNA levels in splenocytes and thymocytes were increased after EPS treatment for 2, 4, 8, or 20 h. EPS also significantly elevated splenic TNF-α and IFN-γ protein expressions at 100 μg mL−1 and increased thymic TNF-α and IFN-γ protein levels at 50 and 100 μg mL−1.82 Cheung et al. (2009) have successfully isolated the novel EPS, namely Cordysinocan with the molecular weight ∼82 kDa which induce cell proliferation and the secretion of interleukin-2, interleukin-6 and interleukin-8. In addition, the phosphorylation of extracellular signal-regulated kinases (ERK) was induced transiently by the treatment of cordysinocan. Moreover, application of cordysinocan in cultured macrophages increased the phagocytosis activity and the enzymatic activity of acid phosphatase, confirming the triggering potential of immune responses.83 The immunomodulating effects of polysaccharides from cultured CS (PCCS) have been evaluated on non-specific and specific immunologic function of immunosuppressed mice. The treatment of PCCS has increased the K and α indices in carbon clearance test, enhanced the phagocytosis function of mononuclear macrophage, murine ear swelling and elevated the hemolysin level in immunosuppressed mice, suggesting that polysaccharides could improve the cellular and humoral immunologic function in immunosuppressed mice.84,85 Administration of CS polysaccharides to LACA mice for 15 days at the dose of 6.85 mg kg−1 not only enhanced delayed type hypersensitivity response but also promoted the plaque forming cell (PFC) response and hemagglutination titers against sheep red cell (SRBC) indicating CS polysaccharides could enhance immune response in mice.86 The polysaccharide treatment caused increase in the weight of thymus gland and improved the delayed hypersensitivity induced by DNFB and also enhanced the phagocytic ability of monocyte macrophages.87
He et al. (2013) isolated a novel protein bound polysaccharide, namely HS002-II, from Hirsutella sinensis with 44 kDa molecular weight and found to enhance the secretion and expression of the cytokines iNOS, TNF-α, IL-1β and NF-κB by HS002-II, which could be developed as a potential immunomodulatory source. The graphical representation shows the IκB-NF-κB pathway behind the immunomodulatory effect of HS002-II in Fig. 4.88 Similar effects were observed with the acid polysaccharide fraction (APSF) treatment on murine macrophage cell line RAW264.7.89 Novel acidic polysaccharides AEPS-1, fractionated from the EPS produced by CS-HK1 fungus in mycelial culture, were treated on Raw264.7 macrophage cell cultures to evaluate the immunomodulatory effect at suitable doses between 25 and 250 g mL−1. The results suggest that the treatment significantly stimulated the release of four major cytokines, TNF-α, IL-1β, IL-6 and IL-10 indicating strong immunostimulatory activity of AEPS-1.7 Polysaccharides from CS play major role in inducing monocyte activation. Among active components crude (CS-P), soluble (CS-Ps) and insoluble (CS-Pp), the macrophage production of TNF-α by CS-Pp was to the highest extent.90 The polysaccharide namely, CS-81002 was found to exhibit immunostimulatory effect on phagocytic function of macrophages in normal mice at dosages of 5 mg kg−1.52 A study demonstrated the potential effect of various extracts of CS mycelium like, petroleum ether extract (PE), ethyl acetate extract (EAE), ethanol extract (EE), glycoprotein (GP) and a purified polysaccharide (PS) on cellular and humoral immune responses of ICR mice against ovalbumin (OVA). The immunized ICR mice were treated with polysaccharides having molecular wt ∼83 kDa at 3 dose levels enhanced the OVA specific IgG, IgG1 and IgG2b antibody in serum to significant levels, concluding these polysaccharides can potentially acts as safe adjuvants.91
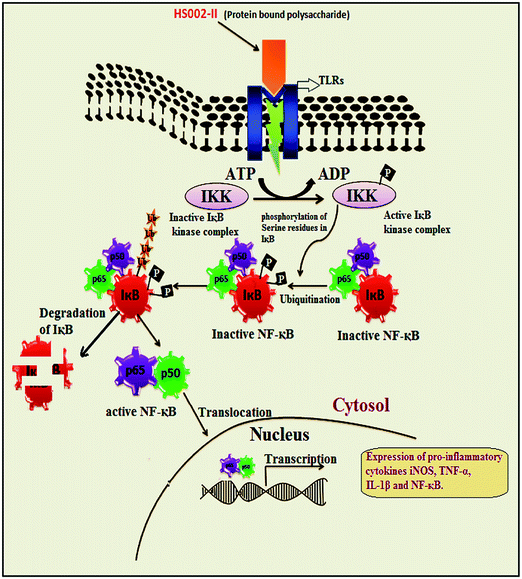 | ||
| Fig. 4 Immuno-stimulation induced via IκB-NF-κB signalling pathway by HS002-II from CS. A study demonstrates the HS002-II polysaccharide treatment of RAW264.7 cells, caused the activation of NF-κB happened via phosphorylation of serine residues and degradation of IκB by IKK. The active NF-κB was translocated into nucleus and modulates gene expressions. NF-κB-nuclear factor kappa-light-chain-enhancer of activated B cells; IKK-IκB kinase; iNOS-inducible nitric oxide synthase; TNF-tumor necrosis factor; IL-interleukin. Adopted from He et al. (2013).88 | ||
Exopolysaccharides fraction (EPSF) from CS mycelium posed activity on B16 melanoma bearing mice and proved to be a potential adjuvant in cancer therapy. The mice were administered with EPSF peritoneally at 3 different doses for 14 times, c-Myc, c-Fos, and VEGF levels in the lungs and livers were found to be significantly lower than those of untreated mice.92 Similar study demonstrated the effect of EPSF on immunocytes of H22 tumor bearing mice where mice were treated by intraperitoneal injection at doses of 15 mg kg−1 (low dose), 30 mg kg−1 (mid dose) and 60 mg kg−1 (high dose). It was observed that EPSF not only significantly inhibited the H22 tumor growth, but also significantly elevated immunocytes activity. It significantly enhanced the phagocytosis capacity of peritoneal macrophages and proliferation ability of spleen lymphocytes at all the three doses; it significantly promoted macrophages TNF-α expression and spleen lymphocytes cytotoxicity. EPSF also significantly elevated TNF-α and IFN-γ mRNA expression of splenic lymphocytes.93 Similar dual effects were observed by others also.94 The effect of EPS from submerged cultured CS on immunomodulatory enhancement of cytokine synthesis, CD11b expression, and phagocytosis was well explained in a study. This immunomodulatory study explored the effect of polysaccharides (Fr. A & Fr. B) on cytokines release, CD11b expression, and phagocytosis in monocytes, PMN, leukocytes. The study concluded that EPS induced the production of tumor necrosis factor alpha (TNF-α), interleukin IL-6, and IL-10 dose dependently. Moreover EPS could significantly augment surface expression of CD11b and has induced phagocytosis in monocytes and polymorphonuclear neutrophils (PMN).95 The graphical representation of immunomodulatory study is depicted in Fig. 5.
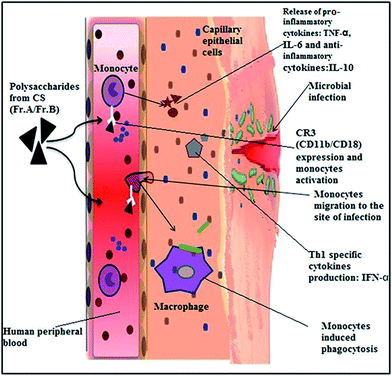 | ||
| Fig. 5 Polysaccharides induced cytokine production, CR3 expression, and phagocytosis. Adopted from Kuo et al. (2007), Sheng et al. (2011), Akaki et al. (2009).82,90,95 | ||
In an in vitro culture system leukemic U937 cells were treated with polysaccharides fraction from CS (PSCS). The study revealed that the conditioned medium with PSCS (10 μg mL−1) stimulated the blood mononuclear cells (PSCS-MNC-CM), significantly inhibiting the proliferation of U937 cells with growth inhibition rate of 78–83%. The treatment of PSCS induced about 50% of the cells differentiating into mature monocytes/macrophages expressing nonspecific esterase (NSE) activity and the surface antigens of CD11b, CD14, and CD68. The levels of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-1 were greatly increased with PSCS stimulation concluding especially IFN-γ and TNF-α acted synergistically on inhibiting cell growth and inducing differentiation of the target U937 cells.96
Heteropolysaccharide from the fruiting bodies of cultured CS (CS-PS) having average molecular weight of 12 kDa were investigated for their effect on immune function of BALB/c mice exposed to 60Co gamma radiation. Mice were administered CS-PS with doses of 50, 100 or 200 mg kg−1 body weight, then exposed to 60Co for four days. The treatment showed significant enhancement in lymphocyte proliferation, the activity of macrophage phagocytosis, DTH and total SOD enzyme activity compared to control group. And also significant reduction in lipid peroxidation level and levels of cytokine IL-4, IL-5 and IL-17 were found to be affected as compared to control group.97 Song et al. (2011) evaluated the effect of EPS from one of the anamorph of CS on murine dendritic cells (DCs). In this experimental study murine DCs were derived from the bone marrow of C57BL/6 mice which are treated with EPS. During the study phenotype molecules, level of phosphorylated signal transducers and activators of transcription 3 (p-STAT3) of DCs were evaluated. The results showed that EPS promoted the levels of surface molecules MHC II, CD40, CD80 and CD86 of DCs and decreased their ingestion ability. The mRNA expressions of cytokines (IL-12p40 and TNF-α) and inducible nitric oxide synthase were up regulated by EPS. It was also found that EPS significantly down regulated p-STAT3 level of DCs. The results concluded that the promotion of DC's maturation and activation by EPS is probably related to the inhibition of STAT3 phosphorylation.98 Potential application of polysaccharide fraction of CS (PSCS) on rapid generation of activated DCs that can be utilized as vaccine for treating chronic myeloid leukaemia (CML). PSCS can increase T cell immunoresponse when CML-DCs incubated by PSCS caused the rapid generation of the co-stimulatory molecules, CD86 and HLA-DR, and the enhancement of IL-12 expression and stimulatory capacity in allogeneic mixed lymphocyte reaction (MLR).99
Polysaccharides from CS were observed to have immune enhanced function in adjuvant arthritis (AA) rats in vitro. Immune cells were treated with CP in order to detect the change of ConA induced splenocyte proliferation, IL-1 and IL-2 synthesis wherein CP (10–100 mg L−1) could not only enhance the reduced ConA-induced splenocyte proliferation, but also improve the decreased IL-2 synthesis in AA rats.100 Furthermore, crude polysaccharides from wild type and mycelia of CS were also observed to induce macrophage from mouse abdominal cavity to produce the tumor necrosis factor (TNF-α).101
5.4 Antitumor effect
Polysaccharides and polysaccharide–protein complexes have been discovered as effective therapies to control human malignancies because chemotherapy drugs cause serious side effects. Many studies have been focused on discovering anticancer polysaccharides and complexes for the development of effective therapeutics for various human cancers.102 Natural polysaccharides from CS are reported to show potent inhibitory effects on many cancers. Intraperitoneal injection of ICR mice with exopolysaccharide fraction (EPSF) from one of the anamorph strains of CS at 15 mg kg−1 (low dose), 30 mg kg−1 (mid dose) and 60 mg kg−1 (high dose) for 7 days showed significant immunocytes activity. But results indicated there was no significant inhibition on tumor growth. It might significantly enhance the phagocytosis capacity of peritoneal macrophages and proliferation ability of spleen lymphocytes at all the three doses. And also it promoted macrophages TNF-α, IFN-γ mRNA expression and spleen lymphocytes cytotoxicity.93 In the similar study, the dual functions of immunomodulatory and antitumor were observed.103 Two novel polysaccharides (α-D-glucans), WIPS (Mw 1180 kDa) and AIPS (Mw 1150 kDa) isolated and characterized from hot and alkaline water extracts of CS mycelium were studied for their antitumor activities.104 Yalin et al. (2005) isolated the cell wall polysaccharides from CS mycelium named as a cordyglucans and tested for their antitumor activities. They concluded that the activity is attributed to β-(1 → 3)-D-glucan linkages.105 Studies also suggested that polysaccharides from CS can be used for the immunotherapy of clinical tumors.106Combination treatment of the polysaccharide rich fraction of CS and cisplatin was tested on H157 NSCLC cells with aim to investigate adjuvant role of CS in the treatment of non-small cell lung cancer (NSCLC). The expression levels of VEGF and bFGF protein were significantly reduced in the cells treated with a combination of CS and cisplatin, concluding CS may be a potential adjuvant chemotherapeutic agent in NSCLC therapy.107 Shen et al. (2009) studied the effects of polysaccharide from CS (PSCS) on triptolide (TPL) induced apoptosis in the HL-60 cells. MTT assays showed that different concentrations of PSCS inhibited the cell viability. Flow cytometry indicated that TPL markedly increased the apoptosis rate of the HL-60 cells, and PSCS enhanced the apoptosis in a dose dependent relationship. Western blot showed that TPL did not inhibit the expression of the Caspase-3, 6, 7, 9 and NF-κB proteins, and when cells were treated with PSCS, the expression of proteins decreased as the PSCS concentration increased. So PSCS can enhance TPL induced apoptosis in HL-60 cells and inhibit the expression of NF-κB and Caspase 3, 6, 7, 9, which might be a possible signalling pathway of inducing apoptosis.108
5.5 Hypoglycaemic effect
Hypoglycaemic effect is a medical emergency that involves drastic reduction in blood glucose content which occurs as a complication of treatment of diabetes mellitus with insulin or oral medication. Recently natural polysaccharides were found to have positive effect on blood glucose reduction. CS polysaccharides can be a potent ingredient possessing hypoglycemic effect. Kiho et al. (1993) studied the effect of polysaccharides on plasma glucose level. Normal mice and streptozotocin induced diabetic mice were treated with crude polysaccharides from fermented CS mycelium by intraperitoneal injection, showed significant response. Crude polysaccharides (CS-OHEP) obtained by alkali extraction method, caused slight reduction in plasma glucose level. A neutral polysaccharide (CS-F30) composed of galactose, glucose and mannose (molar ratio 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with molecular weight of about 45
10) with molecular weight of about 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, exhibited higher hypoglycemic activity than its crude form.109 Effect of CS-F30 polysaccharides on hypoglycemic activity in genetic diabetic mice after intraperitoneal administration was studied. The results revealed that CS-F30 has quickly reduced plasma glucose level which was attributed due to increased hepatic glucokinase, hexokinase and glucose-6-phosphate dehydrogenase activities.110 Another purified polysaccharide fraction (CS-F10) obtained from water extract of CS mycelium having comb type structure and has α-D-glucopyranosyl, β (1 → 5) linked-D-galactofuranosyl residues, could significantly lower the plasma glucose level in normal, streptozotocin (STZ) induced diabetic and epinephrine induced hyperglycemic mice after intraperitoneal administration (50 mg kg−1). The effect is due to increased hepatic glucokinase activity causing reduced hepatic glucose output which was observed following the infusion of CS-F10 using the perfused rat liver.111
000, exhibited higher hypoglycemic activity than its crude form.109 Effect of CS-F30 polysaccharides on hypoglycemic activity in genetic diabetic mice after intraperitoneal administration was studied. The results revealed that CS-F30 has quickly reduced plasma glucose level which was attributed due to increased hepatic glucokinase, hexokinase and glucose-6-phosphate dehydrogenase activities.110 Another purified polysaccharide fraction (CS-F10) obtained from water extract of CS mycelium having comb type structure and has α-D-glucopyranosyl, β (1 → 5) linked-D-galactofuranosyl residues, could significantly lower the plasma glucose level in normal, streptozotocin (STZ) induced diabetic and epinephrine induced hyperglycemic mice after intraperitoneal administration (50 mg kg−1). The effect is due to increased hepatic glucokinase activity causing reduced hepatic glucose output which was observed following the infusion of CS-F10 using the perfused rat liver.111
Purified polysaccharides named as a CSP-1 found to show hypoglycemic activity with antioxidation effect in normal, alloxan diabetic mice and streptozotocin (STZ) diabetic rats. Oral administration of CSP-1 at 200 and 400 mg kg−1 body wt per day for 7 days significantly reduced the blood glucose level by 12.07 ± 3.2% and 22.57 ± 4.7% in normal mice, respectively. In case of both STZ induced diabetic rats and alloxan induced diabetic mice at a dose higher than 200 mg kg−1 body wt daily for 7 days caused significant drop in blood glucose level. The study concluded that CSP-1 has increased insulin level in diabetic animals, which suggests that CSP-1 may stimulate pancreatic release of insulin and/or reduce insulin metabolism.112 Similar studies have been reported the hypoglycemic effect of purified fraction of polysaccharides.113,114 Huang et al. (2002), proposed the mechanism behind the hypoglycemic activity induced by polysaccharides (PCS) from CS mycelium. PCS has raised the glucose uptake in insulin resistant adipocytes.115
5.6 Antioxidative activity
Oxidative stress is a physiological stress on the body that is caused by the cumulative damage done by reactive species and human biological system has an ability to readily detoxify the reactive intermediates or to repair the resulting damage failing which can lead to chronic diseases or sometime fatality. The use of synthetic antioxidants posed many side effects, so natural antioxidants or/and natural products possessing antioxidants are right options with nil side effects. CS is one among them or/and polysaccharides from CS have reported to exhibit potent antioxidative effects.Li et al. (2003) isolated polysaccharides (CSP-1) by using antioxidation activity guided fractionation which have reported to exhibit strong protective effect against hydrogen peroxide (H2O2) induced insult in the cultured rat pheochromocytoma PC12 cells. The treatment of H2O2 at 200 μM reduced the activities of antioxidant enzymes GSH-Px and SOD by 86% and 81%, respectively and pre-treatment with CSP-1 attenuated the changes of GSH-Px and SOD activities in a dose dependent manner. At 100 μg mL−1 of CSP-1, the H2O2 decreased GSH-Px and SOD activities were revered by over 50% and malondialdehyde production also reduced. The study concluded that polysaccharides from CS can provide protection against the free radical induced neuronal cell toxicity.48 Shen et al. (2011) carried out the study where the pheochromocytoma PC12 cells were treated with H2O2 at 300 μM to induce oxidative stress. The oxidative stress was reduced by polysaccharides (APS) treatment which was manifested through changes in activities of GSH-Px, CAT, and SOD, inhibition of ROS accumulation at 100 and 200 μg mL−1 of APS for 24 h, inhibition of intracellular Ca2+ concentration to 133% and 125% and APS significantly inhibited overproduction of MDA.116 Similar studies are also been reported to demonstrate antioxidative activity in literature.28
Four fractions of ethanol extracts of CS, which was fractionated using supercritical CO2 as the elution solvent were characterized to be a polysaccharides and cordycepin showed strong scavenging ability of free radicals. The four fractions at 2 mg mL−1 showed free radical scavenging potency, 93%, 75%, 66%, 47%, and 27%, respectively.117 Dong and Yao (2008) investigated and evaluated the antioxidant potency of polysaccharides isolated from water extract of CS using six in vitro assays. Among these assays, the extracts showed the best effect on the inhibition of linoleic peroxidation with the lowest IC50 values and with an inhibition rate over 90% at concentration of 0.8–1.6 mg mL−1, which proved to be more stable than that of α-tocopherol, a recognized natural antioxidant. The findings demonstrated that superoxide anion and hydroxyl radicals scavenging activities were less than BHT, DPPH assay showed 80% inhibition, finally moderate reducing power and ferrous ion chelating activity.118 Antioxidant potential was evaluated by using xanthine oxidase assay, the induction of haemolysis assay and the lipid peroxidation assay. The study concluded that partial purification of crude polysaccharides enhances the activity by 10 to 30 folds.119
Crude EPS isolated from CS mycelium, are basically polysaccharide protein complexes and were studied for their ability to scavenge radicals and ferric reducing ability of plasma. The results showed that EPS have moderate effect, concluding these biopolymers from the CS mycelial fermentation provide a source of natural antioxidants with potential value for health foods and therapeutics.40 Hydrolysed EPS fractions reported to exhibit high (30–80%) antioxidant and radical scavenging activities.120,121 Polysaccharides (PS) isolated from CS studied for their antioxidative activity against H22 bearing mice, showed that the PS treatment for 9 days had significantly inhibited H22 tumor growth, enhanced SOD activity of liver, brain and serum as well as GSH-Px activity of liver and brain in tumor bearing mice. PS also significantly reduced the level of MDA in liver and brain of tumor bearing mice.24 Heteropolysaccharides from fruiting bodies of cultured CS have effectively reduced oxidative injury to BALB/c mice upon exposure to 60Co. The total SOD enzyme activity in the CS-PS groups was significantly enhanced and lipid peroxidation level was significantly reduced.97 The glucomannogalactan (CPS1), a water soluble polysaccharide has been evaluated for its antioxidant activity by using assays like; hydroxyl radicals scavenging, the reducing power, Fe2+ chelating activity, scavenging effect on superoxide radicals, as well as the inhibition of hydrogen peroxide induced haemolysis. The results concluded that CPS1 showed a high antioxidant effect, especially scavenging effect of hydroxyl radicals, the reducing power and Fe2+ chelating activity.44
Li and Li (2013) studied the enzymatic method of extracting water soluble polysaccharides and evaluation for their antioxidative activity. Water soluble polysaccharides have been extracted by incubating water with 7.5 U or 375 U of cellulase at 50 °C for 2 h. The soluble polysaccharide yield increased 30.38% to 33.23% to 38.10%, while DPPH free radicals scavenging and reducing power were found to be higher than those of untreated control.37 Huang et al. (2013) isolated five EPS fractions P1/5, P2/5, P1, P2 and P5 from the fermentation medium of a medicinal fungus CS by gradient precipitation with ethanol at 1/5, 2/5, 1, 2, and 5 volume ratios to the liquid medium. Each fraction was tested for its antioxidant activity among which P1/5 and P2/5 showed poor response, P1 and P2 showed low to moderate and P5 showed very strong activity.122
5.7 Other
A study was designed to determine the effects of polysaccharides from CS mycelium (CSP) on physical fatigue in mice. The mice were treated with CSP at 100, 200 and 400 mg kg−1, ig for 28 days. Forced swimming test was performed to evaluate endurance capacity of mice and some biochemical parameters related to fatigue were examined. The results showed the exhaustive swimming time of mice for treated group was significantly prolonged as compared to control group.124 The polysaccharides from CS have been evaluated for their protection activity on photoaging skin fibroblasts which were induced by 8-MOP/UVA. CS polysaccharides were administrated before 8-MOP/UVA. HE stained, MTT, hydroxyproline (HYP), MDA and SOD quantitate were used to test the effects of polysaccharides. Cell crimple, condensation of nuclear chromatin in 8-MOP/UVA model group were observed in the study.125 Polysaccharides found to provide protection against photoaging of the dermis layer of skin in mice.126Cordyceps pretreatment has significantly lowered DNA damage in UVB irradiated human fibroblast cells (P < 0.01) after 30 min and 24 h. There was a 27% reduction in cyclobutanepyrimidine dimers (CPDs) in irradiated cells with 24 h pretreatment with 200 mg mL−1 of the hot water extract, and a 34% reduction with 24 h pretreatment with 200 mg mL−1 of the exopolysaccharide extract. Clear evidence of protection against UVB induced CPDs was seen with Cordyceps mycelial extracts. CS may thus offer photoprotection and lower the risk of basal cell carcinoma, the main skin cancer caused by CPDs.127
6. Future prospects
Cordycep sinenis is a medicinal edible mushroom and polysaccharides from CS can undoubtedly be supplemented in ordinary foods, healthy foods, and functional foods. Polysaccharides have been reported non-toxic with very least side effects and can be successfully utilized in cosmetic, food and pharmaceuticals manufacturing industries. Hot water extraction is a popular method for polysaccharides extraction, but other methods like microwave, ultrasound mediated and pressurised liquid extraction techniques are novel and literally very few studies have been reported in literature. Further research activities are required to focus on the above extractions procedures to explore to what extent these can be used effectively. Micro and nano encapsulation techniques are needed to be identified to encapsulate crude polysaccharides or purified fractions of crude polysaccharides from CS. Studies on encapsulation of Cordyceps polysaccharides are necessary to formulate in deliverable forms such as liposomes and food emulsions to engineer sustainable drug delivery systems and to develop health, functional foods and nutraceuticals with unquestionable scientific evidence.Acknowledgements
The author (SMG) acknowledges the award of senior research fellowship from CSIR, New Delhi, India. The authors also acknowledge the Director, CSIR-CFTRI, Mysore, India for the encouragement given.References
- E. T. Öner, Microbial Production of Extracellular Polysaccharides from Biomass, in Pretreatment Techniques for Biofuels and Biorefineries, Springer, Berlin Heidelberg, 2013, pp. 35–56 Search PubMed.
- V. E. COoi and F. Liu, Curr. Med. Chem., 2000, 7, 715–729 CrossRef.
- R. L. Whistler, A. A. Bushway, P. P. Singh, W. Nakahara and R. Tokuzen, Adv. Carbohydr. Chem. Biochem., 1976, 32, 235–275 CrossRef CAS.
- R. Chang, J. Alternative Compl. Med., 2002, 8, 559–565 CrossRef PubMed.
- C. A. Rappleye, L. G. Eissenberg and W. E. Goldman, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 1366–1370 CrossRef CAS PubMed.
- I. Yoshida, T. Kiho, S. Usui, M. Sakushima and S. Ukai, Biol. Pharm. Bull., 1996, 19, 114–121 CAS.
- Z. M. Wang, X. Peng, K. L. D. Lee, J. C. Tang, P. C. K. Cheung and J. Y. Wu, Food Chem., 2011, 125, 637–643 CrossRef CAS PubMed.
- R. Zheng, S. Jie, D. Hanchuan and W. Moucheng, Int. Immunopharmacol., 2005, 5, 811–820 CrossRef PubMed.
- G. Krosl and M. Korbelik, Cancer Lett., 1994, 84, 43–49 CrossRef CAS.
- K. Matsunaga, A. Hosokawa, M. Oohara, N. Sugita, M. Harada and K. Nomoto, Immunopharmacology, 1998, 40, 219–230 CrossRef CAS.
- M. Kanazawa, Y. Mori, K. Yoshihara, M. Iwadate, S. Suzuki, Y. Endoh, S. Ohki, K. Takita, K. Sekikawa and S. Takenoshita, Immunol. Lett., 2004, 91, 229–238 CrossRef CAS PubMed.
- S. Wasser, Appl. Microbiol. Biotechnol., 2002, 60, 258–274 CrossRef CAS PubMed.
- V. Venugopal, Marine Polysaccharides: Food Applications, CRC Press, NY, 2011 Search PubMed.
- T. Mizuno, Int. J. Med. Mushrooms, 1999, 1, 251–261 CrossRef CAS.
- F. Li, X. Gao, B. Rao, L. Liu, B. Dong and L. Cui, Chin. J. Cell. Mol. Immunol., 2006, 22, 321 CrossRef.
- S. Li, F. Yang and K. W. Tsim, J. Pharm. Biomed. Anal., 2006, 41, 1571–1584 CrossRef CAS PubMed.
- CPCMH, Chinese Pharmacopeia, The People's Medical Publishing House, 1963 edn, part one, 1964, p. 77 Search PubMed.
- M. G. Shashidhar, P. Giridhar, K. Udaya Sankar and B. Manohar, J. Funct. Foods, 2013, 5, 1013–1030 CrossRef CAS PubMed.
- X. W. Zhou, L. J. Li and E. W. Tian, Crit. Rev. Biotechnol., 2013, 34, 233–243 CrossRef PubMed.
- H. Kim and J. Yun, J. Appl. Microbiol., 2005, 99, 728–738 CrossRef CAS PubMed.
- C. Hsieh, M. J. Tsai, T. H. Hsu, D. M. Chang and C. T. Lo, Appl. Biochem. Biotechnol., 2005, 120, 145–157 CrossRef CAS.
- S. Cha, J. Lim, C. Yoon, J. Koh, H. Chang and S. Kim, Bioresour. Technol., 2007, 98, 165–168 CrossRef CAS PubMed.
- P. Leung, Q. Zhang and J. Wu, J. Appl. Microbiol., 2006, 101, 275–283 CrossRef CAS PubMed.
- J. Chen, W. Zhang, T. Lu, J. Li, Y. Zheng and L. Kong, Life Sci., 2006, 78, 2742–2748 CrossRef CAS PubMed.
- T. J. Yoon, K. W. Yu, K. S. Shin and H. J. Suh, Appl. Microbiol. Biotechnol., 2008, 80, 1087–1093 CrossRef CAS PubMed.
- X. L. Wang, G. Q. Liu, C. Y. Zhu, G. Y. Zhou and S. M. Kuang, J. Med. Plants Res., 2011, 5, 2873–2878 CAS.
- Y. S. Liu and J. Y. Wu, J. Ind. Microbiol. Biotechnol., 2012, 39, 623–628 CrossRef CAS PubMed.
- L. Wang, G. Wang, J. Zhang, G. Zhang, L. Jia, X. Liu, P. Deng and K. Fan, Carbohydr. Polym., 2011, 86, 1745–1750 CrossRef CAS PubMed.
- J. W. Choi, K. S. Ra, S. Y. Kim, T. J. Yoon, K. W. Yu, K. S. Shin, S. P. Lee and H. J. Suh, Bioresour. Technol., 2010, 101, 6028–6034 CrossRef CAS PubMed.
- P. H. Leung and J. Y. Wu, J. Appl. Microbiol., 2007, 103, 1942–1949 CrossRef CAS PubMed.
- C. Q. Wu, Y. Chen and Y. Hao, Food Sci., 2009, 7, 043 Search PubMed.
- X. Y. Cao, W. Yang, J. L. Lui and H. X. Ai, Adv. Mater. Res., 2013, 726, 401–405 CrossRef.
- J. Ruiz-Herrera, Fungal cell wall: structure, synthesis, and assembly, CRC press, 1992 Search PubMed.
- J. P. Latgé, Mol. Microbiol., 2007, 66, 279–290 CrossRef PubMed.
- S. Nie, S. W. Cui, M. Xie, A. O. Phillips and G. O. Phillips, Bioact. Carbohydr. Diet. Fibre, 2013, 1, 38–52 CrossRef CAS PubMed.
- T. Miyazaki, N. Oikawa and H. Yamada, Chem. Pharm. Bull., 1977, 25, 3324–3328 CrossRef CAS.
- X. L. Li and D. Li, Adv. Mater. Res., 2013, 641, 975–978 Search PubMed.
- X. JianHua, S. Bin and Z. WeiGuo, Chin. J. Bioprocess Eng., 2009, 7, 34–38 Search PubMed.
- Z. M. Wang, Y. C. Cheung, P. H. Leung and J. Y. Wu, Bioresour. Technol., 2010, 101, 5517–5522 CrossRef CAS PubMed.
- P. H. Leung, S. Zhao, K. P. Ho and J. Y. Wu, Food Chem., 2009, 114, 1251–1256 CrossRef CAS PubMed.
- J. K. Yan, L. Li, Z. M. Wang and J. Y. Wu, Carbohydr. Polym., 2010, 79, 125–130 CrossRef CAS PubMed.
- J. Guan, J. Zhao, K. Feng, D. J. Hu and S. P. Li, Anal. Bioanal. Chem., 2011, 399, 3465–3474 CrossRef CAS PubMed.
- D. T. Wu, K. L. Cheong, L. Y. Wang, G. P. Lv, Y. J. Ju, K. Feng, J. Zhao and S. P. Li, Carbohydr. Polym., 2013, 103, 100–109 CrossRef PubMed.
- Y. Wang, M. Wang, Y. Ling, W. Fan, Y. Wang and H. Yin, Am. J. Chin. Med., 2009, 37, 977–989 CrossRef CAS PubMed.
- Z. M. Wang, X. Peng, K. L. D. Lee, J. C. Tang, P. C. K. Cheung and J. Y. Wu, Food Chem., 2011, 125, 637–643 CrossRef CAS PubMed.
- S. W. Cui, Structural analysis of polysaccharides, in Food carbohydrates: chemistry, physical properties, and applications, ed. W. C. Steve, CRC Press, Boca Raton, FL, 1st edn, 2005 Search PubMed.
- S. P. Nie and M. Y. Xie, Food Hydrocolloids, 2011, 25, 144–149 CrossRef CAS PubMed.
- S. P. Li, K. J. Zhao, Z. N. Ji, Z. H. Song, T. T. Dong, C. K. Lo, J. K. Cheung, S. Q. Zhu and K. W. Tsim, Life sci., 2003, 73, 2503–2513 CrossRef CAS.
- S. P. Nie, S. W. Cui, A. O. Phillips, M. Y. Xie, G. O. Phillips, S. Al-Assaf and X. L. Zhang, Carbohydr. Polym., 2011, 84, 894–899 CrossRef CAS PubMed.
- J. Guan, F. Q. Yang and S. P. Li, Molecules, 2010, 15, 4227–4241 CrossRef CAS PubMed.
- S. D. Kim, J. Korean Soc. Appl. Biol. Chem., 2010, 53, 784–789 CrossRef CAS.
- M. Gong, Q. Zhu, T. Wang, X. Wang, J. Ma and W. Zhang, Chin. J. Biochem. Mol. Biol., 1990, 6, 486–492 CAS.
- I. Boukari, J. L. Putaux, B. Cathala, A. Barakat, B. Saake, C. Rémond, M. O. Donohue and B. Chabbert, Biomacromolecules, 2009, 10, 2489–2498 CrossRef CAS PubMed.
- W. Yalin, S. Cuirong and P. Yuanjiang, Carbohydr. Polym., 2006, 63, 251–256 CrossRef PubMed.
- X. Jianming, D. Changhai and L. Liande, Acta Univ. Med. Anhui, 1999, 3 Search PubMed.
- Y. P. Shang, S. Y. Fang, J. F. Ge, L. Zhang and J. Li, West China J. Pharm. Sci., 2007, 22, 654 CAS.
- M. Fan, Acta Univ. Med. Anhui, 1999, 34, 173–175 Search PubMed.
- S. Fang, H. Yao, J. Li, J. Ge, L. Zhang, J. Zhang and M. Fan, Acta Univ. Med. Anhui, 2004, 39, 201–226 Search PubMed.
- X. Ma, D. Qiu, J. Xu and M. Zeng, World Chin. J. Digestol., 1998, 6, 582–584 Search PubMed.
- D. Jing, D. Qiu, S. Xiao, X. Wang and J. Fan, Chin. Hepatol., 1999, 4, 215–216 Search PubMed.
- L. Chunfeng, L. Lei, Y. Li, W. Shuqiu and T. Fushan, Heilongjiang Med. Pharm., 2003, 27, 17–18 Search PubMed.
- J. Peng, X. Li, Q. Feng, L. Chen, L. Xu and Y. Hu, Exp. Biol. Med., 2013, 238, 668–677 CrossRef PubMed.
- F. Li, P. Liu, W. Xiong and G. Xu, China J. Chin. Mater. Med., 2006, 31, 1968 Search PubMed.
- J. Peng, X. Li, Y. Hu and Q. Feng, China J. Chin. Mater. Med., 2013, 38, 391–396 Search PubMed.
- J. L. Yan, H. Li, Y. Fan, J. S. Zhang and F. C. Huang, Fudan Univ. J. Med. Sci., 2003, 30, 27–29 CAS.
- B. W. Fang, P. Liu, C. Liu, Y. Y. Hu, L. M. Xu and F. H. Li, Shanghai J. Tradit. Chin. Med., 2000, 9, 37–40 Search PubMed.
- D. N. Pegler, Y. J. Yao and Y. Li, Mycologist, 1994, 8, 3–5 CrossRef.
- J. S. Zhu, G. M. Halpern and K. Jones, J. Alternative Compl. Med., 1998, 4, 289–303 CrossRef CAS PubMed.
- J. S. Zhu, G. M. Halpern and K. Jones, J. Alternative Compl. Med., 1998, 4, 429–457 CrossRef CAS.
- Y. Wang, H. Yin, X. Lv, Y. Wang, H. Gao and M. Wang, Fitoterapia, 2010, 81, 397–402 CrossRef CAS PubMed.
- W. Q. Fan, H.-p. Yin and C.-l. Zhou, Chin. J. Bioprocess Eng., 2008, 1, 69–73 Search PubMed.
- X. L. Zhang, B. C. Liu, S. Al-Assaf, G. O. Phillips and A. O. Phillips, Food Hydrocolloids, 2012, 28, 200–212 CrossRef CAS PubMed.
- H. Shun-ai, Journal of Taizhou Polytechnic College, 2010, 3, 017 Search PubMed.
- H. P. Yin, X. B. Lv and T. Chen, Tradit. Chin. Drug Res. Clin. Pharmacol., 2007, 18, 451–453 CAS.
- H. P. Yin, X. B. Lv and X. Chen, Chin. J. Bioprocess Eng., 2007, 5, 70–75 CAS.
- X. B. Lv, H. P. Yin, H. T. Li and X. Chen, Prog. Pharm. Sci., 2007, 31, 314–319 Search PubMed.
- T. Chen, H. P. Yin, X. B. Lv and H. Li, Tradit. Chin. Drug Res. Clin. Pharmacol., 2009, 3, 012 Search PubMed.
- S. Guo, F. Zhong, Q. Zhou, Y. Lu, X. Hao, W. Wang and N. Chen, J. Shanghai Jiaotong Univ., 2012, 32, 1–8 Search PubMed.
- M. M. Pan, M. H. Zhang, H. F. Ni, J. F. Chen, M. Xu, A. O. Phillips and B. C. Liu, Food Chem. Toxicol., 2013, 58, 487–494 CrossRef CAS PubMed.
- J. Wu, B. Liu, H. Xia and D. Liu, Chin. J. Integr. Tradit. West. Nephr., 2008, 5, 012 Search PubMed.
- W. Ying, Y. HongPing, C. Tao and W. Min, J. China Pharm. Univ., 2009, 40, 559–564 Search PubMed.
- L. Sheng, J. Chen, J. Li and W. Zhang, Appl. Biochem. Biotechnol., 2011, 163, 669–678 CrossRef CAS PubMed.
- J. K. H. Cheung, J. Li, A. W. H. Cheung, Y. Zhu, K. Y. Z. Zheng, C. W. C. Bi, R. Duan, R. C. Y. Choi, D. T. W. Lau, T. T. X. Dong, B. W. C. Lau and K. W. K. Tsim, J. Ethnopharmacol., 2009, 124, 61–68 CrossRef CAS PubMed.
- R. Jian and Q. L. Zhang, J. Fourth Mil. Med. Univ., 2007, 28 Search PubMed.
- G. Xiaojian, J. Hui, L. Shungao, L. Shaoping and L. Ping, J. China Pharm. Univ., 2000, 31, 53 Search PubMed.
- M. Ling, L. C. Guang and Y. X. Man, J. Health Toxicol., 1995, 3, 162–163 Search PubMed + 176 + 211.
- J. Zhong, Y. Zhang, Z. Ding and K. Ye, Acta Sci. Nat. Univ. Sunyatseni, 2011, 50, 99–102 CrossRef PubMed.
- L. He, P. Ji, J. Cheng, Y. Wang, H. Qian, W. Li, X. Gong and Z. Wang, Food Chem., 2013, 141, 946–953 CrossRef CAS PubMed.
- W. Chen, W. Zhang, W. Shen and K. Wang, Cell. Immunol., 2010, 262, 69–74 CrossRef CAS PubMed.
- J. Akaki, Y. Matsui, H. Kojima, S. Nakajima, K. Kamei and M. Tamesada, Fitoterapia, 2009, 80, 182–187 CrossRef CAS PubMed.
- Y. Wu, H. Sun, F. Qin, Y. Pan and C. Sun, Phytother. Res., 2006, 20, 646–652 CrossRef CAS PubMed.
- J. Yang, W. Zhang, P. Shi, J. Chen, X. Han and Y. Wang, Pathol., Res. Pract., 2005, 201, 745–750 CrossRef PubMed.
- W. Zhang, J. Li, S. Qiu, J. Chen and Y. Zheng, Fitoterapia, 2008, 79, 168–173 CrossRef PubMed.
- W. Zhang, J. Yang, J. Chen, Y. Hou and X. Han, Biotechnol. Appl. Biochem., 2005, 42, 9–15 CrossRef CAS PubMed.
- M. C. Kuo, C. Y. Chang, T. L. Cheng and M. J. Wu, Appl. Microbiol. Biotechnol., 2007, 75, 769–775 CrossRef CAS PubMed.
- Y. J. Chen, M. S. Shiao, S.-S. Lee and S.-Y. Wang, Life Sci., 1997, 60, 2349–2359 CrossRef CAS.
- J. Zhang, Y. Yu, Z. Zhang, Y. Ding, X. Dai and Y. Li, Int. Immunopharmacol., 2011, 11, 2251–2257 CrossRef CAS PubMed.
- D. Song, J. Lin, F. Yuan and W. Zhang, Cell Biochem. Funct., 2011, 29, 555–561 CrossRef CAS PubMed.
- Z. L. Huang, J. Jin, X. M. Tong, Q. Z. Yang, B. L. Gu, J. C. Wang, Y. Shenand and J. J. Xie, J. Med. Plants Res., 2011, 5, 5925–5932 CAS.
- Y. Jin, J. Li, M. Fan, Y. Zhang, C. Li and S. Xu, Acta Univ. Med. Anhui, 2000, 35, 20–21 Search PubMed.
- X. Lin, Y. Zheng, J. Chen and M. Arai, J. Microbiol., 2004, 24, 22–23 CAS.
- A. Zong, H. Cao and F. Wang, Carbohydr. Polym., 2012, 90, 1395–1410 CrossRef CAS PubMed.
- W. Zhang, J. Yang, J. Chen, Y. Hou and X. Han, Biotechnol. Appl. Biochem., 2005, 42, 9–15 CrossRef CAS PubMed.
- J. K. Yan, W. Q. Wang, L. Li and J. Y. Wu, Carbohydr. Polym., 2011, 85, 753–758 CrossRef CAS PubMed.
- W. Yalin, O. Ishurd, S. Cuirong and P. Yuanjiang, Planta Med., 2005, 71, 381–384 CrossRef PubMed.
- Y. R. Zhao, M. L. Wang, B. L. Xu and Z. D. Wu, Basic & Clinical Medicine, 1992, 12, 52–53 Search PubMed.
- N. F. Ji, L. S. Yao, Y. Li, W. He, K. S. Yi and M. Huang, Integr. Cancer Ther., 2011, 10, 359–367 CrossRef CAS PubMed.
- Y. Shen, X. Shao, Y. Ni, H. Xu and X. Tong, J. Zhejiang Univ., Med. Sci., 2009, 38, 158–162 CAS.
- T. Kiho, J. Hui, A. Yamane and S. Ukai, Biol. Pharm. Bull., 1993, 16, 1291–1293 CAS.
- Y. A. Kiho, J. Hui, S. Usui and S. Ukai, Biol. Pharm. Bull., 1996, 19, 294–296 Search PubMed.
- O. K. Kiho, S. Usui, S. Ukai and K. Hirano, Biol. Pharm. Bull., 1999, 22, 966–970 Search PubMed.
- S. Li, G. Zhang, Q. Zeng, Z. Huang, Y. Wang, T. Dong and K. Tsim, Phytomedicine, 2006, 13, 428–433 CrossRef CAS PubMed.
- G. Zhang, Y. Huang, Y. Bian, J. H. Wong, T. Ng and H. Wang, Appl. Microbiol. Biotechnol., 2006, 72, 1152–1156 CrossRef CAS PubMed.
- T. W. Balon, A. P. Jasman and J. S. Zhu, J. Alternative Compl. Med., 2002, 8, 315–323 CrossRef.
- Z. J. Huang, H. Ji, P. Li, L. Xie and X. C. Zhao, J. China Pharm. Univ., 2002, 33, 51–54 CAS.
- W. Shen, D. Song, J. Wu and W. Zhang, Phytother. Res., 2011, 25, 675–680 CrossRef CAS PubMed.
- B. J. Wang, S. J. Won, Z. R. Yu and C. L. Su, Food Chem. Toxicol., 2005, 43, 543–552 CrossRef CAS PubMed.
- C. H. Dong and Y. J. Yao, LWT-Food Sci. Technol., 2008, 41, 669–677 CrossRef CAS PubMed.
- S. Li, P. Li, T. Dong and K. Tsim, Phytomedicine, 2001, 8, 207–212 CrossRef CAS PubMed.
- J. K. Yan, L. Li, Z. M. Wang, P. H. Leung, W. Q. Wang and J. Y. Wu, Food Chem., 2009, 117, 641–646 CrossRef CAS PubMed.
- H. M. Yu, B. S. Wang, S. C. Huang and P. D. Duh, J. Agric. Food Chem., 2006, 54, 3132–3138 CrossRef CAS PubMed.
- Q. L. Huang, K. C. Siu, W. Q. Wang, Y. C. Cheung and J. Y. Wu, Process Biochem., 2013, 48, 380–386 CrossRef CAS PubMed.
- S. H. Wang, W. B. Yang, Y. C. Liu, Y. H. Chiu, C. T. Chen, P. F. Kao and C. M. Lin, J. Lipid Res., 2011, 52, 471–479 CrossRef CAS PubMed.
- J. H. Koh, K. M. Kim, J. M. Kim, J. C. Song and H. J. Suh, Biol. Pharm. Bull., 2003, 26, 691–694 CAS.
- H. Li, T. J. Ye, B. Q. Li and X. P. Ying, Lishizhen Med. Mater. Med. Res., 2009, 20, 1074–1076 CAS.
- H. Li, T. J. Ye, B. Q. Li and X. P. Ying, Lishizhen Med. Mater. Med. Res., 2008, 19, 2679–2681 CAS.
- W. Wong, J. Wu and I. Benzie, Br. J. Dermatol., 2011, 164, 980–986 CrossRef CAS PubMed.
- J. Yuan, X. H. Cheng and Y. Q. Hou, Food Drug, 2005, 7, 45–48 Search PubMed.
- S. Chen, K. C. Siu, W. Q. Wang, X. X. Liu and J. Y. Wu, Carbohydr. Polym., 2013, 94, 332–338 CrossRef CAS PubMed.
- Y. Wu, N. Hu, Y. Pan, L. Zhou and X. Zhou, Carbohydr. Res., 2007, 342, 870–875 CrossRef CAS PubMed.
- T. Kiho, H. Tabata, S. Ukai and C. Hara, Carbohydr. Res., 1986, 156, 189–197 CrossRef CAS.
- M. Soltani, H. Kamyab and H. A. El-Enshasy, J. Pure Appl. Microbiol., 2013, 7, 1–13 Search PubMed.
- H. Ji, H. Tu and N. Li, J. China Pharm. Univ., 1993, 24, 39–42 CAS.
| This journal is © The Royal Society of Chemistry 2015 |