Monolithic porous layer open tubular (monoPLOT) capillary columns for gas chromatography
Ekaterina P. Nesterenkoa,
Maurice Burkeb,
Christophe de Bossetc,
Paulo Pessuttoc,
Claire Malafossec and
David A. Collins*d
aDublin City University, Glasnevin, Dublin 9, Ireland
bNational Centre for Sensor Research, Dublin City University, Glasnevin, Dublin 9, Ireland
cÉcole Nationale Supérieure des Ingénieurs en Arts Chimiques et Technologiques, 31030 Toulouse Cedex 4, France
dIrish Separation Science Cluster, National Centre for Sensor Research, Dublin City University, Glasnevin, Dublin 9, Ireland. E-mail: david.collins@dcu.ie
First published on 23rd December 2014
Abstract
Polymer monolithic open tubular columns are presented as a solid adsorbent for fast and efficient gas phase separations. A porous monolithic layer of polystyrene–divinylbenzene was formed inside a capillary through an in situ polymerisation method creating a long, open bore column with high flow through permeability. The mechanical stability and chromatographic performance of the column was tested, showing the phase to be extremely stable up to 270 °C and capable of fast separations with efficiencies of almost 4000 theoretical plates per meter.
1. Introduction
Monolithic stationary phases can be generally characterised by their continuous rigid structure of interconnected pores and globules that is covalently attached to the inner surface of the column and at capillary scale they are usually formed in situ, making them relatively simple to manufacture. Over the past 15 years there has been growing interest in using organic polymer monoliths as a solid adsorbent in gas chromatography (GC). These materials demonstrate good thermal and chemical stability and can provide a wide range of diversity, both in terms of the chemistry and also morphology of the phase.1 Indeed, several noteworthy studies have been carried out on polymer and silica monolithic phases in GC.3–8 Although fully polymerised monolithic phases have been shown to offer excellent chromatographic performance as a solid absorbent they tend to exhibit a high level of resistance to carrier gas flow with column inlet pressures of up to 200 bar being reported.9 This is beyond the capabilities of the majority of commercial GC instrumentation, and even in cases where modified or bespoke instrumentation has been used, column lengths are limited in order to keep column pressures at a usable level.10–13Since their introduction in 1979, open tubular (OT) columns have become exceedingly popular mainly due to their physical structure, providing very low resistance to carrier gas flow.14 As a result, OT columns can be very long and large numbers of theoretical plates per column can be achieved. However, the reduced amount of stationary phase in OT columns can often lead to column overloading and loss of chromatographic performance.15 A solution to this problem was the introduction of porous layer open tubular (PLOT) columns, which combine the high permeability properties of OT columns with the high surface area of a porous solid material, thus increasing loadability and separating power. The porous structure is usually provided through the static or dynamic deposition of inorganic particles16 or porous polymer beads17,18 on the surface of the capillary, alternatively, the porous coating can be fabricated using in situ polymerisation.19–21 With regard to particle based PLOT phases; static charging during the fabrication process presents a significant problem to the mechanical stability of the column as particles repel one another and can move apart, making the coating unstable. These particles then ‘bleed’ from the stationary phase, plugging columns or even damaging detectors or mass-spectrometers which may be connected to the GC instrument.
Another type of PLOT column which has steadily generated increasing interest over the past 5 years is the monolithic porous layer open tubular (monoPLOT) column. To date, this type of column has mostly found use in various modes of liquid chromatography, such as HPLC, CE and CEC.22 This type of phase structure should be readily extended to GC and has the potential to provide many advantages over existing fully monolithic or PLOT columns. An organic polymer monoPLOT column should also demonstrate good thermal and chemical stability, and since the structure is a rigid, single piece of highly cross-linked polymer, it should also provide superior mechanical stability with minimal column bleed. The challenge in the application of monoPLOT columns to GC lies in the difficulty of their fabrication. Long (>1 m) monoPLOT columns in wide bore capillary (>50 μm ID) are notoriously difficult to manufacture and achieving an acceptable column to column reproducibility is often challenging. Over the past years, several methods for the fabrication of monoPLOT columns have been developed,23–32 most of which focus on smaller (≤50 μm ID) capillaries, however, until recently, it has not been possible to fabricate long monoPLOT columns suitable for GC applications. In this work the authors present the first application of monoPLOT columns to GC and demonstrate the high potential of such a column type in gas phase separations.
2. Experimental
Reagents and materials
All chemicals used within this study were of reagent or analytical grade purity. Styrene, divinylbenzene, 1-decanol, 3-(trimethoxysilylpropyl) methacrylate, and solvents and analytes used for chromatographic evaluation (toluene, ethylbenzene, propylbenzene, butylbenzene, pentylbenzene, methanol, acetonitrile, acetone, 1-propanol, ethyl actate, 1-butanol) were all purchased from Sigma-Aldrich (Gillingham, UK). The thermal initiator, azobisisobutyronitrile (AIBN), was obtained from DuPont (Le Grand Sacconex, Switzerland). All solvents and reagents which were used for the preparation, or for the synthesis and washing of prepared monoliths, namely, sodium hydroxide (NaOH) and hydrochloric acid (HCl), acetonitrile (ACN), were purchased from Lab Scan (Gliwice, Poland). Deionised water was supplied from a Milli-Q system (Millipore, Bedford, MA, USA). Polyimide coated (15 μm coating thickness) fused silica capillary, 200 μm ID, 350 μm OD was purchased from CM Scientific Ltd., Charlestown, UK.Instrumentation
Capillaries were filled with monomer mixture and washed using a KDS-100-CE syringe pump (KD Scientific, Inc., Holliston, MA, USA). Formation of the monolithic layer was carried out in a water bath, using a Yellow Line MST Basic hotplate with TC1 temperature controller and glassware (VWR Ltd., Dublin, Ireland). A Rheodyne 6-port switching valve (Rheodyne, Cotati, CA, USA) was used to switch between the flows of polymerisation mixture and MeOH during the polymerisation process. A SputterCoater S150B (BOC Edwards, Sussex, UK) was used for coating capillary monolithic stationary phase samples with a 30 nm gold layer. Scanning electron microscopy (SEM) analysis was performed on an S-3400N instrument (Hitachi, Maidenhead, UK). Optical microscopy evaluation of samples was performed on a Meiji Techno EMZ-8TR stereomicroscope (Meiji Techno UK Ltd., Somerset, UK). Thermogravimetric analysis (TGA) was performed on a TA Instruments Q50 thermogravimetric analyser (TA Instruments, Newcastle, DE, USA). Porosity and pore size measurements were carried out on a Micromeritics Autopore IV 9500 mercury intrusion porosimeter (Micromeritics, Norcross, GA, USA).Fabrication procedures
Fused silica capillaries were initially pretreated through activation of the surface silanol groups of the inner walls by sequential flushing with 1 M NaOH, deionised water, 0.1 M HCl, deionised water, and acetone. The pretreated capillary was silanised using a 50 %wt solution of 3-(trimethoxysilylpropyl) methacrylate in toluene at 80 °C for 24 hours.The PS-DVB monomer mixture consisted of 8 %wt styrene, 32 %wt divinylbenzene, 18 %wt toluene, 41.5 %wt 1-decanol, and 0.5 %wt AIBN (with respect to monomers). No polymerisation inhibitors were removed and monomers were used as supplied. The initiator (AIBN) was weighed out into the mixture vessel, and the porogen mixture (toluene and 1-decanol) was added, followed by the monomers. The mixture was vortexed and deoxygenated under a flow of nitrogen for 10 min.
The fabrication method for the manufacture of a Ø200 μm ID × 5 m (∼11 μm monolithic phase layer) PS-DVB column was per the procedure described by Collins et al.25 The desired length of silanised capillary (approximately 5.2 m) was coiled and one end connected to a port on the switching valve which was mounted above a heated water bath. The two inlet ports of the switching valve were connected to a syringe filled with polymerisation mixture and another syringe filled with MeOH, respectively. Both syringes were placed in a syringe pump. The coiled capillary was immersed in the water bath and the other end was left open so that the polymerisation mixture could flow through it. The polymerisation mixture was pumped through the capillary at 0.5 mm s−1. After flow was established the water bath was brought up to a polymerisation temperature of 60 °C. The formation of the porous polymer layer was allowed to continue for 3 hours, after which the water bath was evacuated and the hot water was replaced with cold water to quench any further reaction. The switching valve was also switched over to flush the capillary with MeOH in order to remove all unreacted monomer. Once the capillary had been thoroughly washed it was removed and dried under a nitrogen flow for 2 hours. Prior to chromatographic testing the column was conditioned overnight at 270 °C under a flow of nitrogen at 1.0 mL min−1.
Scanning C4D (sC4D) was used to evaluate column homogeneity as per the procedure described by Collins et al.34
Chromatographic procedures
Chromatographic studies on the fabricated column were carried out on an Aglient 7820A gas chromatograph with flame ionisation detection (FID), connected to a PC running EzChrom Elite. The carrier gas in all cases was N2, the flow rate was 0.8 mL min−1, split ratio was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and injection volume was 0.2 μL unless otherwise stated. For the separation of common solvents, including the aqueous mixture, a temperature gradient was run from 0.5 min, heating from 180 °C to 220 °C at 20 °C min−1. The separation of alkylbenzenes was performed isothermally at 270 °C. The mechanical stability study performed on the column was carried out at 270 °C and the column inlet pressure was cycled between 10 and 50 psi (70–350 kPa). The chromatographic stability study was carried out under the same conditions described above for the separation of common solvents, with 50 injections between each recorded chromatogram, over a total of 205 injections on the column.
1, and injection volume was 0.2 μL unless otherwise stated. For the separation of common solvents, including the aqueous mixture, a temperature gradient was run from 0.5 min, heating from 180 °C to 220 °C at 20 °C min−1. The separation of alkylbenzenes was performed isothermally at 270 °C. The mechanical stability study performed on the column was carried out at 270 °C and the column inlet pressure was cycled between 10 and 50 psi (70–350 kPa). The chromatographic stability study was carried out under the same conditions described above for the separation of common solvents, with 50 injections between each recorded chromatogram, over a total of 205 injections on the column.
3. Results and discussion
Thermal stability
TGA was performed to determine the thermal properties of the phase material used in the column. Since GC is carried out at elevated temperatures it was important to first investigate the upper temperature boundary for the methods used in this study so as not to thermally degrade the stationary phase. The plot for the TGA analysis performed on the stationary phase material used in this study is shown in Fig. 1.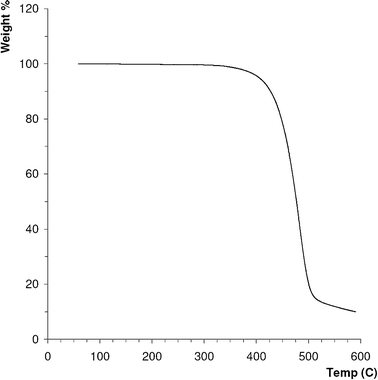 | ||
| Fig. 1 TGA plot for the poly(styrene-divinylbenzene) material used in this study. Heating rate was 20 °C min−1, atmosphere N2. | ||
TGA analysis indicated that the poly(styrene-divinylbenzene) phase used in the column showed good stability up to 300 °C and so the upper temperature used throughout this work was limited to 270 °C. It should be noted however, that other groups have reported higher thermal stabilities for similar materials2 and it is intended to carry out further development of polymer phases with better thermal stabilities in future work.
Layer homogeneity & morphology
Scanning C4D is a very powerful tool for the non-destructive inspection of capillary and micro-bore columns and has been used extensively to examine such columns for various defects, most commonly voids in the phase.33 Recently, Collins et al. further developed this technique further for the in-process measurement of layer thickness and homogeneity within a PLOT column.34 Using this method the layer thickness within the Ø200 μm ID × 5 m column used in this work was found to vary between 9 and 12 μm with a %RSD of approximately 15% (n = 100), see Fig. 2(a). | ||
| Fig. 2 (a) sC4D plot measured along the length of the 5 m column and (b) SEM image of 11 μm PS-DVB layer in a Ø200 μm ID × 5 m capillary column. | ||
An average globule size of 1.6 μm (%RSD = 32%, n = 50) was measured by SEM on sections of capillary removed from each end of the column. An SEM image of a section of the monolithic layer is shown in Fig. 2(b). Average pore size was measured at 8.8 μm using mercury intrusion porosimetry, see Fig. 3.
 | ||
| Fig. 3 Pore size distribution profile (performed by mercury intrusion porosimetry) of the polymer monolithic material used in this study. | ||
Mechanical stability
Since the monolithic layer on the inside of the column is highly porous, the equalisation of gas pressure between the bore of the capillary and within the layer is not instantaneous, and so under rapid pressure changes a pressure differential will exist. If the column pressure or flow rate is suddenly changed it can result in parts of the phase essentially ‘exploding’ as the gas pressure rapidly equalises and indeed this is one of the reasons why PLOT columns are more susceptible to poor reproducibility than liquid phase coated columns. With the increased interest and use of various flow switching techniques in GC this is becoming a real problem for particle based PLOT columns. Rapid pressure cycling can thus be used as a good indication of the mechanical stability of a PLOT column. When the column is coupled to a FID detector during pressure cycling, a series of baseline spikes will indicate particles or pieces of the layer eluting from the column.35 This method was employed to investigate the stability of the monolithic layer within the capillary and a series of fast pressure ramps from 10–50 psi (70–350 kPa) were performed at 270 °C. During this test the FID signal was acquired and is shown in Fig. 4.As can be seen from the plot there is no evidence of ‘spikes’ on the FID signal suggesting that there was no detachment of any part of the phase during the stability study. For reference, an excellent comparison of the impact of a rapid pressure ramp program on both a stable and unstable PLOT column was demonstrated by J. de Zeeuw of Restek Corp.35
GC separation performance
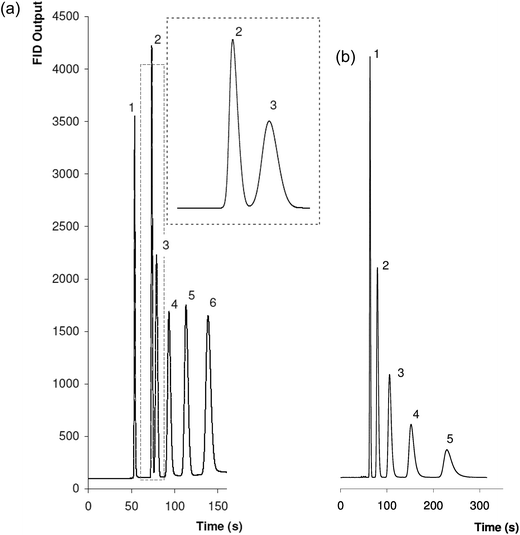
In order to test the chromatographic performance of the fabricated monoPLOT phase several different test mixtures were injected onto the column. Column pressure at 0.8 mL min−1 and 180 °C was recorded at 10.88 psi (75 kPa). Fig. 5(a) shows the separation of a mixture of six common solvents using a temperature gradient from 180 to 220 °C at a ramp rate of 20 °C min−1. Full baseline separation of the analytes is achieved in approximately 2.5 min. As expected, peak asymmetries10% are good given the separation was performed under a temperature gradient; (1.2) methanol, (1.4) acetonitrile, (1.2) acetone, (1.1) 1-propanol, (1.1) ethyl actate, (1.0) 1-butanol. Given that the phase used is 80% divinylbenzene and as such is very hydrophobic, peak elution order confirms that retention from hydrophobic interactions dominates over solvent volatility as the separation mechanism. Other studies have shown similar results for both poly(divinylbenzene)2 and poly(styrene-divinylbenzene)36 columns.An isothermal study for column efficiency based on methanol yielded almost 4000 plates per m (Van Deemter plot is presented in Fig. 6), demonstrating the potential of monoPLOT columns for the fast and efficient separation of small molecules. For comparison, Sýkora et al. performed a similar study on both Ø100 μm and Ø320 μm ID, 50 cm long fully polymerised columns, with the elution of 1-butanol (peak 6 in Fig. 5(a)) after 4.5 min using a temperature gradient of 120 to 300 °C at a ramp rate of 20 °C min−1, reporting a column efficiency of 1600 plates per m for methanol.
 | ||
| Fig. 6 van Deemter plot for methanol using Ø200 μm ID × 5 m (∼11 μm layer) PS-DVB column at 180 °C, carrier gas N2. | ||
Fig. 5(b) demonstrates the fast separation of a mixture of five alkylbenzenes under isothermal conditions at 270 °C in approximately 4.5 minutes. Peak asymmetries10% were acceptable but far from optimal given the isothermal separation conditions and strong interaction of these hydrophobic analytes with the poly(styrene-divinylbenzene) phase; (1.2) toluene, (1.4) ethylbenzene, (1.5) propylbenzene, (1.7) butylbenzene, (1.8) pentylbenzene. Tailing due to column overloading is also possible given the injection volume, nonetheless, the column loadability is significantly higher than for other OT column types. Even with mediocre peak shape, full baseline separation of the alkylbenzene mixture was achieved.
A chromatographic stability study of the column (see Fig. 7(a)) was performed over a period of 3 months and 205 injections of different analytes with various temperature programs. This was achieved by performing the same injection of the mixture of common solvents (as per Fig. 5(a)) every 50th injection under the same conditions (180–220 °C at 20 °C min−1, flow rate 0.8 mL min−1). Any column deterioration over this period would result in retention time shift, particularly for later eluting peaks, however, the column was found to be exceptionally stable, with the retention time of 1-butanol varying by as little as 1% over the course of the study. As well as the mechanical stability of the column, this further shows the poly(styrene-divinylbenzene) phase to be highly chemically stable and inert. Another advantage of this type of monolithic phase is its insensitivity to aqueous samples. The presence of water in a sample can cause many issues in GC analysis, particularly in cases where non-bonded phases are used.37 In their work on GC separations on a fully monolithic column, Sýkora et al. demonstrated excellent column stability with several injections of a 10% aqueous sample at 180 °C.2
 | ||
| Fig. 7 (a) Repeated injections of (1) methanol, (2) acetonitrile, (3) acetone, (4) 1-propanol, (5) ethyl actate, and (6) 1-butanol carried out over a 3 month period (205 injections). Variation in tr for 1-butanol is approximately 1%. (b) Three chromatograms showing the separation of a 20% aqueous mixture of the analytes used in Fig. 5(a) and (a), made over a total of 30 injections, (i) 10th injection, (ii) 20th injection, (iii) 30th injection. | ||
In theory, an OT structure of the same or similar phase should be even less prone to degradation from aqueous samples as there is a much smaller likelihood that water (or any of the sample for that matter) will penetrate through the layer to the capillary wall. This is simply due to the fact that the path of least resistance for the gas flow is along the open bore of the column, and not through the comparably smaller pore structure of the phase itself. Additionally, ‘backflash’ (which is also a concern with aqueous samples) should not present a problem in an OT column as there is ample volume to accommodate the rapid expansion. To demonstrate the suitability of this type of column to the separation of aqueous samples, 30 injections of a 20% aqueous sample containing the same solvent mixture as before were performed on the column. These separations were also carried out using the same chromatographic conditions (180–220 °C at 20 °C min−1, flow rate 0.8 mL min−1). Three representative chromatograms made after 10, 20, and 30 injections are shown in Fig. 7(b). No shift in peak retention times is observed and they are identical to the non-aqueous samples. Peak symmetries also remain unchanged, albeit with some slight fronting on the methanol peak.
The observed results from this relatively short monoPLOT column show that this type of phase is extremely promising for GC separations. Further work which will investigate the effect of layer thickness and morphology of the separation performance is currently underway and it is hoped that this in turn will lead to greater chromatographic evaluation and application of longer columns, columns of different diameter and with different stationary phase chemistries and functionality.
4. Conclusions
The work presented in this preliminary study demonstrates the suitability of monolithic porous layer open tubular phases as solid adsorbents in gas chromatography. Although various types of porous polymer phase exist for GC in an open tubular structure, this work represents the first example of an open tubular polymer monolithic column to be used in gas phase analysis. The developed phase has been shown to be chemically inert and demonstrates excellent mechanical stability and performance over 200 injections. In addition to this, organic polymer monoliths of the type used in this work can be easily tailored in terms of surface chemistry, allowing the user to fine tune parameters such as polarity and selectivity. Importantly, this type of column also exhibits high flow through permeability with a reasonable level of efficiency giving fast analysis times with good resolution. Future work will provide an in-depth study of the effect of layer thickness and morphology on the separation performance.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to acknowledge financial support from Science Foundation Ireland for the Irish Separation Science Cluster Award (Grant Number 08/SRC/B1412) and also from Enterprise Ireland for the Commercialisation Fund Award (CF 2013 3022).References
- F. Svec, J. Chromatogr., 2010, 1217, 902 CrossRef CAS PubMed
.
- D. Sykora, E. C. Peters, F. Svec and J. M. J. Frechet, Macromol. Mater. Eng., 2000, 275, 42 CrossRef CAS
.
- A. Kurganov, Anal. Chim. Acta, 2013, 775, 25 CrossRef CAS PubMed
.
- A. A. Korolev, V. E. Shiryaeva, T. P. Popova, A. V. Kozin, I. A. D'yachkov and A. A. Kurganov, Polym. Sci. Ser. A, 2006, 48, 779 CrossRef
.
- A. A. Korolev, V. E. Shiryaeva, T. P. Popova and A. A. Kurganov, Russ. J. Phys. Chem. A, 2010, 84, 1617 CrossRef CAS
.
- A. A. Korolev, V. E. Shiryaeva, T. P. Popova and A. A. Kurganov, Russ. J. Phys. Chem., 2005, 79, 457 CAS
.
- A. A. Korolev, V. E. Shiryaeva, T. P. Popova and A. A. Kurganov, Russ. J. Phys. Chem., 2006, 80, 120 CrossRef CAS
.
- E. N. Viktorova, A. A. Korolev, T. R. Ibragimov and A. A. Kurganov, Polym. Sci., Ser. A, 2012, 54, 5385 CrossRef
.
- Q. Tang, Y. Shen, N. Wu and M. L. Lee, J. Microcolumn Sep., 1999, 11, 415 CrossRef CAS
.
- A. A. Korolev, V. E. Shiryaeva, T. P. Popova and A. A. Kurganov, Russ. J. Phys. Chem., 2006, 80, 1135 CrossRef CAS
.
- A. V. Kozin, A. A. Korolev, V. E. Shiryaeva, T. P. Popova and A. A. Kurganov, Russ. J. Phys. Chem. A, 2008, 82, 276 CAS
.
- A. A. Korolev, V. E. Shiryaeva, M. E. Dianov, T. P. Popova and A. A. Kurganov, Pet. Chem., 2012, 52, 437 CrossRef CAS
.
- A. A. Korolev, V. E. Shiryaeva, T. P. Popova and A. A. Kurganov, Russ. J. Phys. Chem. A, 2013, 87, 120 CrossRef CAS
.
- R. D. Dandeneau and E. H. Zerenner, HRC & CC, J. High Resolut. Chromatogr. Chromatogr. Commun., 1979, 1, 351 Search PubMed
.
- A. A. Korolev, T. P. Popova, V. E. Shiryaeva, A. V. Kozin and A. A. Kurganov, Russ. J. Phys. Chem. A, 2007, 81, 469 CrossRef CAS
.
- C. F. Poole, S. K. Poole, Chromatography Today, Elsevier, Amsterdam, 5th edn, 1991, p. 1026 Search PubMed
.
- O. H. Hollis, Anal. Chem., 1966, 38, 309 CrossRef CAS
.
- R. L. Grob, Modern Practice of Gas Chromatography, Wiley-VCH, New York, 3rd edn, 1995, p. 912 Search PubMed
.
- J. De Zeeuw, R. C. M. De Nijs, J. C. Buyten, J. A. Peene and M. Mohnke, J. High Resolut. Chromatogr., 1998, 11, 162 CrossRef
.
- T. C. Shen, J. Chromatogr. Sci., 1992, 30, 239 CAS
.
- T. C. Shen and M. M. Fong, J. Chromatogr. Sci., 1994, 32, 36 CAS
.
- D. A. Collins, E. P. Nesterenko and B. Paull, Analyst, 2014, 139, 1292 RSC
.
- G. Yue, Q. Luo, J. Zhang, S. Wu and B. L. Karger, Anal. Chem., 2007, 79, 938 CrossRef CAS PubMed
.
- I. Nischang, O. Brueggemann and F. Svec, Anal. Bioanal. Chem., 2010, 397, 953 CrossRef CAS PubMed
.
- D. A. Collins, E. P. Nesterenko, D. Brabazon and B. Paull, Chromatographia, 2013, 76, 581 CAS
.
- Z. Walsh, S. Abele, B. Lawless, D. Heger, P. Klan, M. Breadmore, B. Paull and M. Macka, Chem. Commun., 2008, 6504 RSC
.
- S. Eeltink, F. Svec and J. M. J. Frechet, Electrophoresis, 2006, 27, 4249 CrossRef CAS PubMed
.
- S. Abele, P. Smejkal, O. Yavorska, F. Foret and M. Macka, Analyst, 2010, 135, 477 RSC
.
- E. Nesterenko, O. Yavorska, M. Macka, A. Yavorskyy and B. Paull, Anal. Methods, 2011, 3, 537 RSC
.
- D. A. Collins, E. P. Nesterenko, D. Brabazon and B. Paull, Anal. Chem., 2012, 84, 3465 CrossRef CAS PubMed
.
- C. Ecoffet, A. Espanet and D. Lougnot, J. Adv. Mater., 1998, 10, 411 CrossRef CAS
.
- D. A. Collins, E. P. Nesterenko and B. Paull, RSC Adv., 2014, 4, 28165 RSC
.
- E. Gillespie, D. Connolly, M. Macka, P. N. Nesterenko and B. Paull, Analyst, 2007, 12, 1238 RSC
.
- D. A. Collins, E. P. Nesterenko, D. Brabazon and B. Paull, Analyst, 2013, 138, 2540 RSC
.
- J. de Zeeuw, LC-GC Europe, 2011, 38 CAS
.
- Z. Ji and R. E. Majors, LC-GC, 1998, 16, 620 CAS
.
- E. R. Kuhn, LCGC Asia Pacific, 2002, 5(3), 30 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |