Improving SO2 capture by tuning functional groups on the cation of pyridinium-based ionic liquids†
Abstract
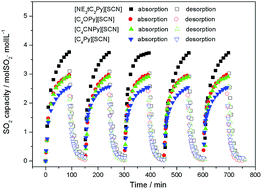
In this work, three kinds of novel functionalized ionic liquids (ILs) [NEt2C2Py][SCN], [C4OPy][SCN] and [C4CNPy][SCN] were developed by introducing a tertiary amino group, ether group and nitrile group on the pyridinium cation to improve SO2 absorption performances. Among the investigated ILs, [NEt2C2Py][SCN] showed the highest absorption capacity of 1.06 gSO2 gIL−1 under ambient conditions due to a combination of chemical and physical absorption. By contrast, the enhancement in SO2 capacity by [C4CNPy][SCN] and [C4OPy][SCN] is mainly ascribed to the stronger physical interaction between ILs and SO2 than the conventional IL [C4Py][SCN]. Meanwhile, higher SO2/CO2 selectivity was also obtained using these functionalized ILs, which was increased up to 41% comparing with that of [C4Py][SCN]. Moreover, the effect of water on SO2 capacity and the absorption mechanism were studied. The results indicated that the presence of water caused a slight decrease in SO2 capacity of [C4CNPy][SCN] and [C4OPy][SCN] because of physical absorption, whereas a slight increase in SO2 capacity by [NEt2C2Py][SCN] due to the formation of hydrogen sulfite salts through chemical absorption. In addition, three kinds of cation-functionalized ILs could remain the stable absorption performance after five cycles of absorption and desorption, implying these ILs show great potentials for SO2 capture.
- This article is part of the themed collection: Ionic Liquids: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...