Introducing a protective interlayer of TiO2 in Cu2O–CuO heterojunction thin film as a highly stable visible light photocathode†
Abstract
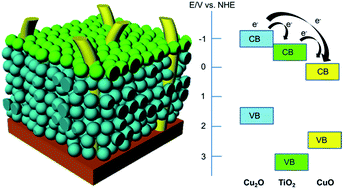
Visible light-induced photocurrent generation and photoelectrochemical stability of p-type Cu2O–CuO photocathodes are improved significantly upon incorporating an interlayer of TiO2 between Cu2O and CuO. The TiO2 layer hinders the electron conduction at the semiconductor–electrolyte interface (improved stability) as well as promoting electron transfer from Cu2O to CuO (increased photocurrent). Upon visible light illumination, the optimised multilayer Cu2O–TiO2–CuO heterojunction thin film yields a photocurrent of 2.4 mA cm−2 and retains 75% of its photoactivity over the measurement period. By comparison, the unmodified Cu2O–CuO generates a photocurrent of 1.3 mA cm−2 with photoactivity retention of only 32% after prolonged illumination. Wavelength-dependent incident photon-to-current efficiency (IPCE) reveals a considerable enhancement over the excitation region of Cu2O (400–560 nm). Transient fluorescence decay analysis suggests the promotion of electron transfer from Cu2O to CuO through TiO2. As a result, both photoactivity and photochemical stability of the photocathodes are improved.

 Please wait while we load your content...
Please wait while we load your content...