Synthetic application of gold nanoparticles and auric chloride for the synthesis of 5-substituted 1H-tetrazoles†
Satyanand Kumara,
Arvind Kumara,
Alka Agarwal*b and
Satish Kumar Awasthi*a
aChemical Biology Laboratory, Department of Chemistry, University of Delhi, Delhi-110007, India. E-mail: satishpna@gmail.com; Tel: +91 1127666646 ext. 121, 134
bDepartment of Medicinal Chemistry, IMS, BHU, Varanasi-221005, India. E-mail: alka_ag_2000@yahoo.com; Tel: +91 9453048577
First published on 13th February 2015
Abstract
An effective one-pot, convenient gold catalyzed synthesis of 5-substituted 1H-tetrazoles has been discussed. The study demonstrated the comparative overview for utilization of gold(III) and gold nano-particles (spheres) as a catalyst. Detailed understandings of the mechanism, surface area effect (in reference to nanoparticles) of gold in the activation of nitriles for nucleophilic (3 + 2) cycloaddition of sodium azide to give the product have also been studied.
1. Introduction
The 1H-tetrazole functional group is a versatile moiety in organic synthesis and is found to be quite stable in a broad pH range, and has good tolerance towards various oxidizing and reducing agents.1 It is important pharmacophore in the field of medicinal chemistry2 and its pharmaceutical applications are vastly explored and comprehensively documented in the literature.3 The tetrazole-based drug candidates possess anticonvulsants,4 antihypertensive, antiallergic and antibiotic activities,5 and have shown promising results in treatment of dreaded diseases, such as cancer and AIDS.6 Besides, tetrazoles are broadly used in agriculture as herbicides and fungicides.7 It is also reported that biphenyltetrazoles stimulate the release of growth hormones,8 and thus widely used to synthesize sartan family drugs.9 Furthermore, tetrazoles are also used in photography and photo-imaging as stabilizers.2 The tetrazoles are lipophilic, metabolically stable compounds10 and can be considered for carboxylic acid bioisosteres,11 cis-amide isosteres in peptide chemistry12 as well as in material sciences.13 Owing to the high enthalpy of formation, their derivatives have been explored in various explosives and propellant components.14 Further, tetrazoles have low molecular weight and possess high nitrogen content,15 and can possess various substituents to form stable complexes with several metal ions,16 which makes it important intermediate in coordination chemistry.17 Tetrazoles are selectively synthesized by (3 + 2) dipolar cycloaddition of an azide moiety over nitrile through a concerted and regioselective18 manner to give 5-substituted 1H-tetrazoles. 5-Substituted 1H-tetrazoles synthesis is fascinating field in organic synthesis, and several new methods have emerged since its synthesis by Finnegan, which are either revision of existing processes or new catalytic protocols.19 The synthesis involves the influence of several efficient catalysts and different solvent conditions. Till date, several new catalysts have also been investigated. These catalysts, which serve the aforementioned purpose, are copper triflates,20 zinc(II) salts,21 Fe(OAc)2,22 Lewis acids, such as FeCl3,23 TBAF,24 AlCl3,25 BF3–OEt2,26 heterogeneous catalysis, CoY zeolites,27 mesoporous ZnS nanospheres,28 Cu2O29 and CuFe2O4 nano particles.30 Acid catalysts are also used for the synthesis of tetrazoles via cycloaddition.31 However, majority of previously reported procedures, which involve Lewis acids, were found poor catalysts for the synthesis of aliphatic tetrazoles or they have not included the synthesis of aliphatic tetrazole.19 Furthermore, they were found riddled with several potential drawbacks like long reaction time, elevated temperature conditions (120–190 °C),32 low yield, use of toxic metals. In addition to this, some cases show formation of toxic hydrazoic acid,33 involvement of alkyltin based reagents which warrant the safety of the procedure. The importance of tetrazoles in the field of medicinal chemistry demands newer, safe and sustainable protocols for the synthesis of tetrazoles with minimal drawbacks associated with previous methods. Thus, the choice of catalyst is one of the most crucial steps to achieve good results.Over the past few decades, transition metals have attracted the attention of chemists, and consequently several reports have shown the utility of transition elements and their salts as a catalyst in organic synthesis. Being a soft Lewis acid,34 gold has attracted considerable attention of chemists for its potential use as catalysis. The potential catalytic property of gold is attributed to its well known π-acidity35 and thus exploited well in recent years to influence a range of transformations. Many organic transformations, such as coupling reactions,36 Friedel Crafts type,37 cycloaddition reactions,38 cyclization of allenol and alkynol,39 carbon–carbon bond formation,36 C–H activation,40 from C–X (X = O, N) bond formation are known to be catalyzed by Au(III) state, and this enhanced catalytic activity is attributed to their Lewis acidity.41 Similarly, AuNPs feature a wide range of potential applications in various fields such as catalysis,42 biotechnology,43 and medicine,44 and are thus may be explored as the most powerful tool for studying nanoscale materials. This broad range of applicability of gold encourages us to use it as a catalyst for the synthesis of 5-substituted 1H-tetrazoles. The extensive literature survey reveals that till date, neither AuNPs nor HAuCl4·3H2O as catalysts have been used in the synthesis of 5-substituted 1H-tetrzole. Herein, we are going to report the utility of gold nano particles and auric chloride for one pot synthesis of 5-substituted 1H-tetrazoles via (3 + 2) cycloaddition with high yields. Our research is focused on design and synthesis of biologically active molecules45 and X-rays analysis of small molecules.46 In diversification of our ongoing research, we recently reported new catalytic method for synthesis of carboxylic acids47 and tetrazoles.48
2. Experimental
2.1 General experimental condition for HAuCl4·3H2O catalysed reaction
The representative tetrazole 1b was synthesized via following procedure: sodium azide (1.5 mmol) was added to a solution of nitrile 1a (1 mmol) and HAuCl4·3H2O (10 mmol%) in dry DMF (2 mL) and reaction mixture was stirred for 0.45 h at 110 °C under nitrogen atmosphere. After consumption of 1a as seen by TLC, the reaction mixture was cooled to room temperature and the DMF was evaporated under reduced pressure. The crude reaction mixture was dissolved in ethyl acetate (30 mL) and washed with acidified water (20 mL, 4 N HCl) twice. The organic layer was separated and washed with brine solution, dried over anhydrous Na2SO4, and solvent was evaporated till dryness to obtained tetrazole 1b as a white crystalline solid in 90% yield.2.2 General experimental condition for Au nano-spheres (AuNPs) catalysed reaction
Sodium azide (1.5 mmol) was added to a solution of nitrile 1a (1 mmol) and AuNPs (2 mmol%) in anhydrous DMF (2 mL). The reaction mixture was stirred for 0.3 h at 80 °C under nitrogen atmosphere. After consumption of 1a as seen by TLC, the reaction mixture was cooled to room temperature and solvent evaporated under reduced pressure. The crude reaction mixture was dissolved in ethyl acetate (30 mL) and washed with acidified water (20 mL, 4 N HCl) twice. The organic layer was separated and washed with brine solution dried over anhydrous Na2SO4, and solvent was evaporated to obtained tetrazole 1b as a white crystalline solid in 95% yield. All synthesized compounds were characterized by using various analytical techniques viz. melting point measurements, 1H, 13C NMR, IR and X-rays (12b Au(III) as a catalyst and 14b by using AuNPs catalysed procedure) crystallography study (Fig. 1) and the data were found comparable with the literature reported data.2.3 General experimental procedure for synthesis of Au nano-spheres (AuNPs)
Gold nano particles were synthesized according to the reported literature procedure.49 Its morphology and particles size was determined by using UV-vis absorption spectrum, DLS and TEM, techniques (Fig. 2–5). UV-vis absorption spectrum of the synthesized AuNPs exhibited a strong absorption peak around 526 nm, which was found dependent on the size and shape of particles (Fig. 3). Size distribution analysis by DLS showed average particle size around 26.33 nm (Fig. 4). Furthermore, the size and shape analysis of AuNPs by TEM imaging technique showed uniform distribution with predominantly spherical morphology with their average size of about 26 ± 3 nm (Fig. 2).To ascertain the oxidation state of Au(0) in AuNPs, we performed XPS experiment. Briefly, X-rays photo electron spectroscopic measurements were carried out by using VG Micro Tech ESCA-3000. AlKα X-ray radiation (hν = 1486.6 eV) with a spectral resolution of 0.4 eV. Analyzer-mode passes energy of 50 eV. The spectrum was charge calibrated with respect to the adventitious C1s peak at 285.0 eV.
The XPS analysis of AuNPs confirmed that the Au is in zero oxidation state (Fig. 6). The values exactly match with earlier reported spectra of Au(0).50 Interpretation of spectra reveals two peaks at binding energy 84.0 eV and 87.7 eV corresponding to Au4f7/2 and Au4f5/2, respectively. The Au4f region has well separated spin–orbit coupling components with binding energy difference of 3.7 eV, which is a characteristic of Au(0).
3. Results and discussion
The broad spectrum of utilities of gold in chemical transformations plays major role in catalysis. This catalytic activity is supposedly attributed to its ability of activation of a particular functionality via coordination, which selectively affords the reactivity of the molecule. For example, our result explicitly shows involvement of activation of nitrile functionality of cyanobenzene by an Au(III or 0) state of catalyst. The pre-coordination of nitrile functional group with Au(III or 0) state, activates nitrile moiety for the (3 + 2) cycloaddition nucleophilic reaction with sodium azide. This enhanced activation of nitrile with Au(III) is probably due to its Lewis acid behaviour. Furthermore, the Au(0) state, in case of AuNPs, facilitates the same coordination chemistry owing to its negative zeta potential (Fig. 5) which, along with its large surface area available for coordination, enhance its Lewis acidity and therefore, leads to substantial yield of tetrazole formation. Functional compatibility of Au(III and 0) catalysts, for several functional groups like halides, alkoxy, amines, nitro, methyl, hydroxy and aldehydes were found well tolerated and preserved during the tetrazole formation. Besides, the AuNPs catalysed reaction shows better yield which may be the outcome of large surface area. This may also be accounted for the small reaction time and comparatively low temperature requirements (75 °C) with low catalyst loading (2 mmol%).In the current study, we investigated the effect of other solvents, such as DMF, NMP, DMSO, DCM, acetonitrile, chloroform, acetone, 1,4-dioxane, and EtOH, on account of their solubility, ease of product formation, effect on yield and environmental compatibility. Among the solvents tested, DMF was found most efficient solvent on many aspects, (Table 1, entries 1–4), while no detectable reaction was observed in chloroform, DCM, 1,4-dioxane and acetone even after prolonged heating under optimized conditions of catalyst (Table 1, entries 11–14). Further, in acetonitrile and ethanol, only small amount of product formed (5–7%, Table 1, entries 9 and 10) and, in case of DMSO, NMP and toluene conversion was achieved up to 56–60% at elevated temperature under optimized conditions of catalyst (10 mmol% and 110 °C) (Table 1, entries 5, 6 and 8).
| Entry | Catalyst (mmol%) | Solvent | Temp. (°C) | Time (h) | Yield (%) 1b |
|---|---|---|---|---|---|
| 1 | 5 | DMF | 110 | 1.00 | 75 |
| 2 | 10 | DMF | 110 | 0.45 | 90 |
| 3 | 10 | DMF | 110 | 1.30 | 91 |
| 4 | 15 | DMF | 110 | 1.30 | 93 |
| 5 | 10 | DMSO | 110 | 8.00 | 56 |
| 6 | 10 | NMP | 110 | 3.00 | 60 |
| 7 | 15 | NMP | 110 | 3.00 | 60 |
| 8 | 10 | Toluene | 110 | 3.00 | 58 |
| 9 | 10 | CH3CN | 80 | 22.0 | 7 |
| 10 | 10 | EtOH | 78 | 12.0 | 5 |
| 11 | 10 | CHCl3 | 61 | 24.0 | 0 |
| 12 | 10 | DCM | 39 | 24.0 | 0 |
| 13 | 10 | 1,4-Dioxane | 100 | 24.0 | 0 |
| 14 | 10 | Acetone | 56 | 24.0 | 0 |
The optimization of catalysts were achieved by setting a number of experiments via varying the quantity of HAuCl4·3H2O and AuNPs, using 1a as a starting material, shown in Tables 1 and 2 respectively. The best yield (93%) was obtained in conversion of 1a to 1b with 15 mmol% of HAuCl4·3H2O in DMF as a catalyst (Table 1, entry 4), while conversion reduced to 75% at 5 mmol% catalyst (Table 1, entry 1). Further, setting the reaction with amount of HAuCl4·3H2O up to 10 mmol% resulted in small decrease in yield (90%) with almost negligible effect (Table 1, entry 2). Moreover, prolonged heating (1.3 h) does not show significant increase in the yield (91%, Table 1, entry 3). Optimization of reaction temperature, to achieve maximum yield, was found out by carrying reaction at various temperatures, and eventually (100–110 °C) with Au(III) and 75–80 °C with Au(0) were found optimal temperature. DMF was found best solvent with respect to temperature, time and catalyst loading parameters. This optimized protocol (1 equiv. nitrile, 1.5 equiv. NaN3, and 10 mmol% of HAuCl4·3H2O in DMF at 100–110 °C while in case of AuNPs it was 2 mmol% at 75–80 °C) consistently yielded good amount of products in all cases (Table 3). Furthermore, the protocol was also found applicable for aliphatic tetrazole formation in appreciable good yield (Scheme 1 and 2).
| Entry | Catalyst AuNPs (Fig. 2–5) | Temp (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1. | Au(0) (1 mmol%) | 80 | 1.30 | 80 |
| 2. | Au(0) (2 mmol%) | 80 | 0.30 | 95 |
| 3. | Au(0) (5 mmol%) | 80 | 1.00 | 95 |
| 4. | Au(0) (10 mmol%) | 80 | 1.30 | 96 |
| S. no. | Nitrilesa | Tetrazoles | Temp (°C) Au(III)/Au(0) | Time (h) Au(III)/Au(0) | % Yields Au(III)b | % Yields Au(0)c |
|---|---|---|---|---|---|---|
| a Nitriles 1 (1a–16a except 9a) and NaN3 (1.5 equiv.) were subjected to react in dry DMF containing 10 mmol% of HAuCl4·3H2O/2 mmol% AuNPs, at given temperature and time details are given in the Table 3.b Experimental yield.c Experimental yield.d Nitriles 9a was reacted with NaN3 (3.0 equiv.) in dry DMF containing 20 mmol% of HAuCl4·3H2O/4 mmol% AuNPs at given temperature and time.e Fig. 1.f Fig. 1. | ||||||
| 1 |  |
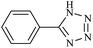 |
110/80 | 0.45/0.30 | 90 | 95 |
| 2 | 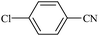 |
 |
100/75 | 1.30/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
90 | 91 |
| 3 |  |
 |
100/80 | 2.5/2.00 | 90 | 92 |
| 4 |  |
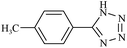 |
110/80 | 2/1.50 | 96 | 98 |
| 5 |  |
 |
100/80 | 1.10/1.00 | 98 | 99 |
| 6 |  |
 |
100/80 | 0.55/0.45 | 89 | 91 |
| 7 |  |
 |
110/80 | 1.00/1.00 | 92 | 93 |
| 8 |  |
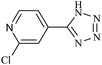 |
110/80 | 1.00/1.00 | 95 | 96 |
| 9d | 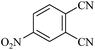 |
 |
110/80 | 1.30/1.20 | 94 | 96 |
| 10 |  |
 |
100/80 | 1.40/1.30 | 89 | 94 |
| 11 | 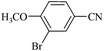 |
 |
110/80 | 0.30/0.30 | 92 | 94 |
| 12e | 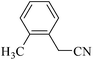 |
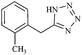 |
110/80 | 1.00/1.00 | 82 | 84 |
| 13 | 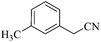 |
 |
110/80 | 1.00/1.00 | 86 | 85 |
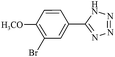
| 14f |  |
 |
110/80 | 1.00/1.00 | 85 | 87 |
| 15 |  |
 |
110/80 | 1.00/1.00 | 82 | 83 |
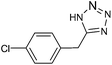
| 16 | 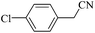 |
 |
100/80 | 1.20/1.10 | 84 | 84 |
The results of DMF mediated (3 + 2) cycloaddition reaction of various nitriles [a] with NaN3, using 10 mmol% HAuCl4·3H2O (with 49% purity) or 2 mmol% AuNPs as a catalyst for the formation of their respective tetrazoles are summarized in Table 3. It is observed that the reactions of the aryl nitriles 4a and 5a, bearing an electron-donating group at the para-position of the aromatic ring possess better yield (Table 3, entries 4 and 5) than electron withdrawing (Table 3, entry 6) group when present at para position. The effect of slightly deactivating group were also studied and found only small drop in yield (Table 3, entries 2 and 3). Further, steric crowding has no significant effect in the synthesis of tetrazoles (Table 3, entry 7). This method is also significant for heteroaromatic tetrazole formation from their corresponding heteroaromatic nitriles (Table 3, entry 8). In addition to this, it is also found applicable for aliphatic nitrile transformation (Table 3, entries 12–16). Dicyano derivative also converted into ditetrazole without any selectivity (Table 3, entry 9). Besides, the reaction is also tested on disubstituted nitriles, and the results indicate that meta substituent has no role while para substitution with electron releasing group to gives intermediate yield (Table 3, entries 10 and 11). The above results stipulate that the tetrazole ring formation via (3 + 2) cycloaddition reaction involving Au(III) and Au(0) do not affect the other substituents and functional group and it is well tolerable irrespective of their electronic behaviour, positions and independent of the type of aromatic or aliphatic ring.
3.1 Leaching experiment
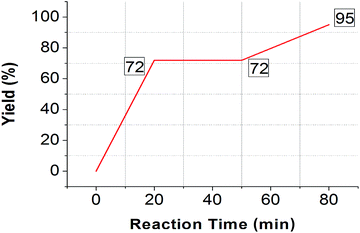
For getting the best results and to avoid any discrepancy, three parallel reactions were performed in separate apparatus, but at the same time and under similar reaction conditions. In first reaction mixture, the tetrazole synthesis was conducted at 80 °C for 20 minutes in the presence of catalyst which yielded 72% of product after usual work up.Simultaneously in second reaction, the reaction was conducted for 20 minutes and the AuNPs were removed from the reaction mixture by filtration. The reaction mixture was allowed to run for another 30 minutes at 80 °C. No progress of the reaction in this span was observed as the yield remains constant after work up (72%).
In the third experiment, the reaction was followed the same protocol up to 50 min as mentioned in 2nd experiment then, AuNPs were added in desired catalytic amount to the reaction mixture and reaction was allowed to stir for another 10 minutes which gave quantitative yield of product after workup (95%) (Scheme 1, Fig. 7).
These experiments confirmed that AuNPs are essential for completion of reaction. The heterogeneous nature of the catalyst was confirmed by ICP-OES analysis (Spectro Arcos, FHS-12).
No leaching of Au metal was detected in the ICP analysis of reaction mixtures. We conclude that AuNPs in reaction medium act as heterogeneous catalyst. ICP data analysis is shown in Table 5 in ESI.†
In order to establish superior approach for the tetrazoles synthesis, we compared with other well known existing method for tetrazoles synthesis. It is evident from Table 4 that use of AuNPs/HAuCl4·3H2O gave high yield at lower temperature and in lesser reaction time as compared to others. Thus we can conclude that AuNPs/HAuCl4·3H2O are better catalyst for tetrazoles synthesis.
| S. no. | Catalyst | Reaction Time (h) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| a Present method. | ||||
| 1 | HAuCl4·3H2Oa | 0.45 | 110 | 90 |
| 2 | AuNPsa | 0.30 | 80 | 95 |
| 3 | Cu–Zn alloy nanopowder15 | 10 | 120 | 95 |
| 4 | ZnBr2 (ref. 21) | 24 | Reflux | 76 |
| 5 | Fe(OAc)2 (ref. 22) | 24 | 80 | 56 |
| 6 | FeCl3–SiO2 (ref. 23) | 12 | 120 | 79 |
| 7 | TMSN3·TBAF·3H2O24 | 18 | 85 | 86 |
| 8 | CoY zeolite27 | 14 | 120 | 90 |
| 9 | Mesoporous ZnS nanospheres28 | 36 | 120 | 96 |
| 10 | CuFe2O4 (ref. 30) | 12 | 120 | 82 |
| 11 | SiO2–H2SO4 (ref. 31) | 12 | 130 | 88 |
| 12 | Amberlyst-15 (ref. 33) | 12 | 85 | 85 |
4. Mechanism
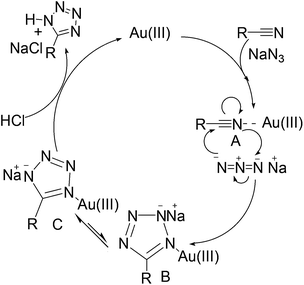
The uses of gold in catalytic organic transformations are well known because of its electrophilic activation. The π-electron-containing compounds, such as alkenes, alkynes, allenes, facilitate this auration.51 Here in this report, the given starting molecule is supposed to be activated via the auration of nitrile functionality for transformation into respective tetrazole. A plausible mechanism for the synthesis of 5-substituted 1H-tetrazole as shown in Scheme 3, is Au(III) coordinate initially with nitrile group through its π-electron-cloud which assist to activate C–N functionality to form intermediate [A] for nucleophilic addition with NaN3, which generates the intermediate [B]. The reaction proceeds via (3 + 2) cycloaddition between the C–N bond of nitrile and NaN3. The complex [B] rearranges to produce more stable [C] which on protonolysis by 35% HCl (pH of solution was adjusted in between 2 and 3) affords the desired product 5-substituted 1H-tetrazole and Au(III) at the end of the reaction. Similar mechanism is expected for Au(0) nanoparticle catalysed reaction, the enhanced reactivity of Au nanoparticles might be due to large surface area which facilitates more coordination between C–N of nitrile and Au(0).5. Conclusions
We developed a newer highly efficient method for the synthesis of 5-substituted 1H-tetrazoles by treatment of sodium azide with various functionalized nitriles. This functionalization of nitriles was achieved by using auric chloride in gold(III) state and gold nanospheres as gold(0) state for catalysis. In addition to aromatic and heteroaromatic tetrazole synthesis, the protocol is found working efficiently for the aliphatic tetrazoles synthesis with good yield, which shows its superiority over other previously reported procedures. In the previously reported cases, low loading of the gold catalyst is sufficient for catalysis and the requirement of short reaction time is competitive with the cost-effective alternatives. The significant advantages of this methodology include simple work-up procedure, easy preparation and handling of the catalyst, high yields, elimination of dangerous and harmful hydrazoic acid formation and no column chromatography of the final product. This report may open a new avenue of reactivity in synthetic organic chemistry.X-ray crystallographic study
X-Ray data were collected on Crysalis PRO (Oxford Diffraction, 2009) with graphite mono75 chromate MoKα radiation (λ = 0.71073 Å) at 298(2) K. The structure was solved by a direct method using SHELXL-97 and refined by full matrix least-squares method on F2 (SHELXL-97).12b Crystal data for shelxl: C9H10N4, M = 174.21, monoclinic, a = 7.8232(8) Å, b = 11.9434(14) Å, c = 9.8716(9) Å, α = 90.00°, β = 96.825(8)°, γ = 90.00°, V = 915.82(17) Å3, T = 298(2) K, space group P21/c, Z = 4, μ(MoKα) = 0.082 mm−1, 6368 reflections measured, 1788 independent reflections (Rint = 0.0221). The final R1 values was 0.0619 (I > 2σ(I)). The final wR(F2) values was 0.1981 (I > 2σ(I)). The final R1 values was 0.0734 (all data). The final wR(F2) values was 0.2061 (all data). The goodness of fit on F2 was 1.450.14b Crystal data for shelxl: C9H10N4, M = 174.21, monoclinic, a = 14.907(2) Å, b = 4.9488(5) Å, c = 12.804(2) Å, α = 90.00°, β = 108.31(2)°, γ = 90.00°, V = 896.8(2) Å3, T = 298(2) K, space group P21/c, Z = 4, μ(MoKα) = 0.084 mm−1, 4341 reflections measured, 2427 independent reflections (Rint = 0.0312). The final R1 values were 0.0759 (I > 2σ(I)). The final wR(F2) values were 0.2288 (I > 2σ(I)). The final R1 values was 0.1224 (all data). The final wR(F2) values was 0.2931 (all data). The goodness of fit on F2 was 0.923.Acknowledgements
Satyanand Kumar and Arvind Kumar are thankful to the UGC, New Delhi, India for award of JRF. AA is thankful to the UGC, New Delhi, India and Banaras Hindu University, Varanasi, for financial support. SKA is thankful to the University of Delhi, Delhi for financial assistance. Authors are also thankful to A. S. Nagpure and Dr Satyanarayana Chilukuri for ICP analysis and Dr Kashinath Patil, NCL Pune, India for providing XPS facility.Notes and references
- G. F. Holland and J. N. Pereira, J. Med. Chem., 1967, 10, 149 CrossRef CAS.
- M. L. T. Frija, A. Ismael and M. L. S. Cristiano, Molecules, 2010, 15, 3757 CrossRef PubMed.
- R. S. Upadhayaya, S. Jain, N. Sinha, N. Kishore, R. Chandra and S. K. Arora, Eur. J. Med. Chem., 2004, 39, 579 CrossRef CAS PubMed.
- A. D. Sarro, D. Ammendola, M. Zappala, S. Grasso and G. B. D. Sarro, Antimicrob. Agents Chemother., 1995, 39, 232 CrossRef.
- (a) T. Mavromoustakos, A. Kolocouris, M. Zervou, P. Roumelioti, J. Matsoukas and R. Weisemann, J. Med. Chem., 1999, 42, 1714 CrossRef CAS PubMed; (b) J. H. Toney, P. M. Fitzgerald, N. Grover-Sharma, S. H. Olson, W. J. May, J. G. Sundelof, D. E. Vanderwall, K. A. Cleary, S. K. Grant, J. H. Wu, J. W. Kozarich, D. L. Pompliano and G. G. Hammond, Chem. Biol., 1998, 5, 185 CrossRef CAS; (c) Y. Hashimoto, R. Ohashi, Y. Kurosawa, K. Minami, H. Kaji, K. Hayashida, H. Narita and S. Murata, J. Cardiovasc. Pharmacol., 1998, 31, 568 CrossRef CAS PubMed.
- (a) A. D. Abell and G. J. Foulds, J. Chem. Soc., Perkin Trans. 1, 1997, 1, 2475 RSC; (b) Y. Tamura, F. Watanabe, T. Nakatani, K. Yasui, M. Fuji, T. Komurasaki, H. Tsuzuki, R. Maekawa, T. Yoshioka, K. Kawada, K. Sugita and M. Ohtani, J. Med. Chem., 1998, 41, 640 CrossRef CAS PubMed.
- K. Kubendiran, J. Raguraman, S. R. M. Kamil and S. S. Shafi, Asian J. Res. Chem., 2011, 4, 1915 Search PubMed.
- L. Demange, D. Boeglin, A. Moulin, D. Mousseaux, J. Ryan, G. Berge, D. Gagne, A. Heitz, D. Perrissoud, V. Locatelli, A. Torsello, J. C. Galleyrand, J. A. Fehrentz and J. Martinez, J. Med. Chem., 2007, 50, 1939 CrossRef CAS PubMed.
- G. Wang, B. Sun and Z. Ru, Synth. Commun., 2008, 38, 3577 CrossRef CAS.
- Z. P. Demko and K. B. Sharpless, Org. Lett., 2001, 3, 4091 CrossRef CAS PubMed.
- (a) R. J. Herr, Bioorg. Med. Chem., 2002, 10, 3379 CrossRef CAS; (b) A. J. A. Cobb, D. A. Longbottom, D. M. Shaw and S. V. Ley, Chem. Commun., 2004, 1808 RSC.
- J. Zabrocki, G. D. Smith, J. B. Dunbar Jr, H. Iijima and G. R. Marshall, J. Am. Chem. Soc., 1988, 110, 5875 CrossRef CAS.
- (a) Z. P. Demko and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2110 CrossRef CAS; (b) J. X. Y. Luo, L. L. Zhao, C. H. Wang and J. S. Wu, Inorg. Chem. Commun., 2013, 31, 23 CrossRef PubMed.
- Z. P. Demko and K. B. Sharpless, J. Org. Chem., 2001, 66, 7945 CrossRef CAS PubMed.
- G. Aridoss and K. K. Laali, Eur. J. Org. Chem., 2011, 6343 CrossRef CAS.
- (a) Y. Tang, G. Wang, Q. Ye, R. Xiong and R. Yuan, Cryst. Growth Des., 2007, 7, 2382 CrossRef CAS; (b) B. Li, S. Chen, Z. Chen, J. Chen, J. Guo and L. Liu, CrystEngComm, 2011, 13, 6610 RSC.
- R. J. Hert, Bioorg. Med. Chem., 2002, 10, 3379 CrossRef.
- R. Huisge, J. Org. Chem., 1968, 33, 2291 CrossRef.
- J. Roh, K. Vávrová and A. Hrabálek, Eur. J. Org. Chem., 2012, 6101 CrossRef CAS.
- L. Bosch and J. Vilarrasa, Angew. Chem., 2007, 46, 3926 CrossRef CAS PubMed.
- Z. P. Demko and K. B. Sharpless, J. Org. Chem., 2001, 66, 7945 CrossRef CAS PubMed.
- J. Bonnamour and C. Bolm, Chem.–Eur. J., 2009, 15, 4543 CrossRef CAS PubMed.
- M. Nasrollahzadeh, Y. Bayat, D. Habibi and S. Moshaee, Tetrahedron Lett., 2009, 50, 4435 CrossRef CAS PubMed.
- D. Amantini, R. Beleggia, F. Fringuelli, F. Pizzo and L. Vaccaro, J. Org. Chem., 2004, 69, 2896 CrossRef CAS PubMed.
- D. P. Matthews, J. E. Green and A. J. Shuker, J. Comb. Chem., 2000, 2, 19 CrossRef CAS PubMed.
- A. Kumar, R. Narayanan and H. Shechter, J. Org. Chem., 1996, 61, 4462 CrossRef CAS.
- V. Rama, K. Kanagaraj and K. Pitchumani, J. Org. Chem., 2011, 76, 9090 CrossRef CAS PubMed.
- L. Lang, B. Li, W. Liu, L. Jiang, Z. Xu and G. Yin, Chem. Commun., 2010, 46, 448 RSC.
- (a) T. Jin, F. Kitahara, S. Kamijo and Y. Yamamoto, Tetrahedron Lett., 2008, 49, 2824 CrossRef CAS PubMed; (b) T. Jin, F. Kitahara and Y. Yamamoto, Chem.–Asian J., 2008, 3, 1575 CrossRef CAS PubMed.
- B. Sreedhar, A. S. Kumar and D. Yadav, Tetrahedron Lett., 2011, 52, 3565 CrossRef CAS PubMed.
- Z. Du, C. Si, Y. Li, Y. Wang and J. Lu, Int. J. Mol. Sci., 2012, 13, 4696 CrossRef CAS PubMed.
- P. B. Palde and T. F. Jamison, Angew. Chem., Int. Ed., 2011, 50, 3525 CrossRef CAS PubMed.
- (a) D. P. Curran, S. Hadida and S. Y. Kim, Tetrahedron, 1999, 55, 8997 CrossRef CAS; (b) R. Shelkar, A. Singh and J. Nagarkar, Tetrahedron Lett., 2013, 54, 106 CrossRef CAS PubMed.
- A. H. Roder and N. Krause, Org. Biomol. Chem., 2005, 3, 387 Search PubMed.
- (a) A. Furstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (b) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed.
- H. A. Wegner and M. Auzias, Angew. Chem., Int. Ed., 2011, 50, 8236 CrossRef CAS PubMed.
- M. T. Reetz and K. Sommer, Eur. J. Org. Chem., 2003, 3485 CrossRef CAS.
- F. Gasparrini, M. Giovannoli, D. Misiti, G. Natile, G. Palmieri and L. Maresca, J. Am. Chem. Soc., 1993, 115, 4401 CrossRef CAS.
- B. Alcide and P. Almendros, Acc. Chem. Res., 2014, 47, 939 CrossRef PubMed.
- (a) T. C. Boorman and I. Larrosa, Chem. Soc. Rev., 2011, 40, 1910 RSC; (b) A. H. Roder and N. Krause, Org. Biomol. Chem., 2005, 3, 387 RSC.
- H. C. Shen, Tetrahedron, 2008, 64, 7847 CrossRef CAS PubMed.
- (a) G. C. Bond, C. Louis and D. T. Thompson, Catalysis by Gold, Imperial College Press, London, 2006, vol. 6 Search PubMed; (b) B. S. Takale, M. Bao and Y. Yamamoto, Org. Biomol. Chem., 2014, 12, 2005 RSC.
- J. J. Storhoff, R. Elghanian, R. C. Mucic, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 1959 CrossRef CAS.
- (a) J. A. Copland, M. Eghtedari, V. L. Popov, N. Kotov, M. Mamedova, M. Motamedi and A. A. Oraevsky, Mol. Imag. Biol., 2004, 6, 341 CrossRef PubMed; (b) N. Khlebtsov and L. Dykmana, Chem. Soc. Rev., 2011, 40, 1647 RSC.
- (a) M. K. Singh, R. Tilak, G. Nath, S. K. Awasthi and A. Agarwal, Eur. J. Med. Chem., 2013, 63, 635 CrossRef CAS PubMed; (b) S. K. Dixit, N. Mishra, M. Sharma, S. Singh, A. Agarwal, S. K. Awasthi and V. K. Bhasin, Eur. J. Med. Chem., 2012, 51, 52 CrossRef CAS PubMed.
- S. Singh, M. K. Singh, A. Agarwal and S. K. Awasthi, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, 01616 Search PubMed.
- S. Kumar, S. K. Dixit and S. K. Awasthi, Tetrahedron Lett., 2014, 55, 3802 CrossRef CAS PubMed.
- S. Kumar, S. Subey, N. Saxena and S. K. Awasthi, Tetrahedron Lett., 2014, 55, 6034 CrossRef CAS PubMed.
- N. N. Long, L. V. Vu, C. D. Kiem, S. C. Doanh, C. T. Nguyet, P. T. Hang, N. D. Thien and L. M. Quynh, J. Phys.: Conf. Ser., 2009, 187, 012026 CrossRef.
- (a) J. Sylvestre, S. Poulin, A. V. Kabashin, E. Sacher, M. Meunier and J. H. T. Luong, J. Phys. Chem. B, 2004, 108, 16864 CrossRef CAS; (b) Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261 CrossRef CAS PubMed; (c) B. Kong, J. Geng and H. Jung, Chem. Commun., 2009, 2174 RSC; (d) Z. Yujie, G. Make, Z. Huan, H. Yao, P. Cheng, H. Qing and F. Chunhai, Chin. Sci. Bull., 2012, 57, 3086 CrossRef.
- (a) N. D. Shapiro and F. D. Toste, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2779 CrossRef CAS; (b) R. Skouta and C. J. Li, Tetrahedron, 2008, 64, 4917 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: All spectroscopic data of compounds. CCDC 929716 and 951458. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra13461c |
| This journal is © The Royal Society of Chemistry 2015 |