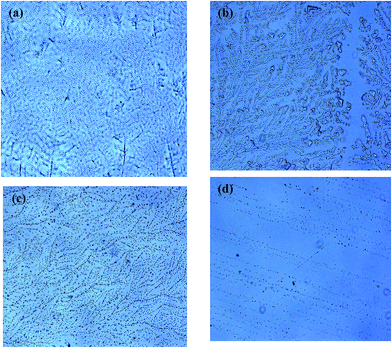
In vitro cytocompatibility and blood compatibility of polysulfone blend, surface-modified polysulfone and polyacrylonitrile membranes for hemodialysis
Anirban Roya,
Prabhash Dadhichb,
Santanu Dharab and
Sirshendu De*a
aDepartment of Chemical Engineering, Indian Institute of Technology, Kharagpur P.O. Box 721302, India. E-mail: sde@che.iitkgp.ernet.in; Fax: +91-3222-255303; Tel: +91-3222-283926
bSchool of Medical Science and Technology, Indian Institute of Technology, Kharagpur P.O. Box 721302, India
First published on 15th December 2014
Abstract
The fabrication of dialysis membranes with significant biocompatibility is an active area of research. In this context, three types of asymmetric flat sheet membranes were fabricated and compared for potential use as hemodialysis membranes. A polysulfone–polyvinylpyrrolidone and polyethylene glycol-based polymer blend membrane, a polysulfone membrane surface-modified with trimesoyl chloride and m-phenylene diamine, and a polyacrylonitrile membrane were synthesized. All three types of membrane were characterized in terms of their surface morphology, permeability, hydrophilicity, surface charge, porosity and mechanical strength. They were then subjected to comprehensive cytocompatibility and hemocompatibility tests as well as analysing the transport of uremic toxins. On the basis of protein adsorption, oxidative stress, cell proliferation and adhesion, all three membranes were comparable. However, the blend and surface-modified membranes showed excellent results for hemolysis, platelet adhesion, blood cell aggregation and degree of thrombus formation. All these results indicated the suitability of the blend and surface-modified membranes for possible dialysis applications.
1. Introduction
Dialysis in the wake of kidney failure, or acute kidney injury (AKI),1–3 is a lifeline for survival of the patient. Replacing the kidney function with a membrane module in an extracorporeal circuit has resulted in shifting the focal point of nephrological research to the development of biocompatible membranes. In the past several decades, importance has been given to polymeric materials, blends and surface modifications to achieve better dialysis-grade membranes with greater efficacy and improved biocompatibility. In this endeavour, cellulose acetate (CA) has been used since the 1850’s, following the pioneering work of Graham4 and Fick,5 exhibiting its potential in ultrafiltration and dialysis. However, a whole gamut of problems, viz. low flux6 and complement activation,7,8 was encountered by practising clinicians using CA membranes. Ever since the Artifical Kidney-Chronic Uremia program was launched by NIAMD (National Institute of Arthritis and Metabolic Diseases) in the year 1966, materials scientists and engineers have started looking into the possibility of developing synthetic polymers for dialysis with significant hemocompatibility.This led to the emergence of different materials with improved hemocompatibility. Therefore, polyacrylonitrile9–11 (PAN) followed by polysulfone12–14 (PSf) based membranes started to gain popularity in hemodialysis applications. Polyethersulfone (PES) blended with citric acid-grafted polyurethane15 has been reported as a possible dialysis membrane. Carbon nanotube-grafted PES composite membranes,16 chemically modified PSf,17 vitamin E–TPGS (D-α-tocopheryl polyethylene glycol 1000 succinate) composite membranes,18 and polyamide and monosodium glutamate blend membranes have also been explored for dialysis applications.19,20 However, biocompatibility is not the only issue for the selection of membranes by practising clinicians. The development of high-performance membranes has become a pivotal issue in dialysis treatment, since higher and faster clearance of uremic toxins increases the patients’ longevity.21,22 In fact, PSf-based membranes have a higher clearance rate for uremic toxins23 and have become a prime choice for clinicians administering dialysis, taking into account both its biocompatibility and transport properties.24
In view of the above discussion, it is clear that variants of PSf and PAN are suitable materials for hemodialysis applications. Therefore, the present article details an attempt to formulate three types of dialysis grade membranes based on PSf and PAN, and to evaluate their in vitro cell and blood compatibility as well as uremic toxin transport capabilities. The underlining step in synthesizing these membranes is attaining a specific molecular weight cut-off (MWCO) of around 6–16 kDa. Here, this was achieved through three different methodologies. First, a polymer blend membrane (S1) was synthesized using polysulfone (PSf) as a base material, blended with polyvinylpyrrolidone (PVP) and polyethylene glycol (PEG) with 6 kDa MWCO. Second, a PSf and PVP blend membrane (S2) was synthesized via surface treatment using trimesoyl chloride (TMC) and m-phenylene diamine (MPD) to yield a similar cut-off. Interestingly, TMC and MPD are in widespread use in synthesizing reverse osmosis membranes,25 but have not yet been reported for their potential use in dialysis membranes. Lastly, a polyacrylonitrile (PAN) homopolymer membrane (S3) was prepared with a dialysis grade MWCO.26 All the membranes were characterized for permeability, hydrophilicity, porosity, membrane morphology and mechanical strength. Detailed cytocompatibility and hemocompatibility analyses were carried out to evaluate biological activity. Finally, the performances of the membranes were quantified in terms of their urea and creatinine permeances. The results were interpreted and a final recommendation was made in terms of performance. Therefore, the novelty of this work includes the formulation of PSf–PVP–PEG, surface modified PSf–PVP and PAN homopolymer membranes and exploring their suitability for hemodialysis purposes.
2. Materials and methods
2.1 Membrane synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da, supplied by Solvay Chemicals, Mumbai, India), PVP (1, 2 and 3 wt%, molecular weight 40
400 Da, supplied by Solvay Chemicals, Mumbai, India), PVP (1, 2 and 3 wt%, molecular weight 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, supplied by Sigma Aldrich, Missouri, USA) and PEG ( 3 wt%, supplied by S R Ltd., Mumbai, India) were dissolved in dimethyl formamide (DMF) (supplied by Merck (India) Mumbai Ltd.), by stirring over a magnetic stirrer (supplied by Anupam enterprises, Kharagpur, India) at around 60 °C for over 10 h. The solution was then kept overnight for degassing and was cast the next day over a non-woven fabric support (118 ± 22.8 μm thickness, supplied by Hollytex, India Inc., New York, USA). A film of thickness 150 μm was cast using a doctor’s blade (fabricated and supplied by Gurpreet Engineering Works, Kanpur, India).
000 Da, supplied by Sigma Aldrich, Missouri, USA) and PEG ( 3 wt%, supplied by S R Ltd., Mumbai, India) were dissolved in dimethyl formamide (DMF) (supplied by Merck (India) Mumbai Ltd.), by stirring over a magnetic stirrer (supplied by Anupam enterprises, Kharagpur, India) at around 60 °C for over 10 h. The solution was then kept overnight for degassing and was cast the next day over a non-woven fabric support (118 ± 22.8 μm thickness, supplied by Hollytex, India Inc., New York, USA). A film of thickness 150 μm was cast using a doctor’s blade (fabricated and supplied by Gurpreet Engineering Works, Kanpur, India).2.2 Membrane characterization
 | (1) |
Polyethylene glycol (PEG) of various molecular weights was supplied by S R Ltd., Mumbai, India. The molecular weights were 1000, 4000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 20
000, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 70
000, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 100
000 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, and they are essentially neutral polymers. A 10 kg m−3 solution of each, prepared by separately dissolving the polymers in distilled water, was fed to a stirred batch cell.26 A low transmembrane pressure (70 kPa) and high stirring speed (2000 rpm) were applied to minimize the concentration polarization layer, and the permeate was collected at intervals of five minutes and the percentage rejection (%R) measured:
000 Da, and they are essentially neutral polymers. A 10 kg m−3 solution of each, prepared by separately dissolving the polymers in distilled water, was fed to a stirred batch cell.26 A low transmembrane pressure (70 kPa) and high stirring speed (2000 rpm) were applied to minimize the concentration polarization layer, and the permeate was collected at intervals of five minutes and the percentage rejection (%R) measured:
 | (2) |
 | (3) |
 | (4) |
 | (5) |
2.3 Biological assessments of membranes
For in vitro biological assessments, NIH3T3 (mouse embryonic fibroblast cell line) cells were procured from the National Centre for Cell Science (NCCS) Pune, India. NIH3T3 cells were grown up to confluence in medium containing alpha-modified essential medium (αMEM) (12561-056, Invitrogen Life Sciences, India) with 1% antibiotics, antimyotic solution (penicillin 100 μg ml−1, streptomycin 10 μg ml−1, and amphotericin-B 25 μg ml−1; A002A Himedia, India) and 10% fetal bovine serum (Himedia, India), at 37 °C, 95% humidity and 5% CO2 (Heracell150i, Thermo, USA).For the assessment of biological activity, the membranes were cut with identical dimensions, sterilized and soaked in cell culture medium overnight. For each experiment, 1 × 104 cells per cm2 were seeded on the samples and a control in a 12 well cell culture plate. Subsequently, the required volume of medium was added to each well and cultured for a particular time interval with respect to the assay type. All the assays were performed in triplicate and their mean values reported. Commercially available dialysis fiber (Fresenius F6) was used as a control in the experiments.
On the respective day of the assay, the cell-seeded membranes were rinsed with phosphate buffered saline (PBS), further incubated with 200 μl of 5 mg ml−1 MTT solution (M5655, Sigma), in the dark under standard cell culture conditions. The dehydrogenase enzymes of metabolically active cells reduced the pale yellow MTT reagent to soluble purple-colored formazan crystals. The formazan product was dissolved in dimethyl sulfoxide (DMSO) and the absorbance was measured at 570 nm on a microplate reader (Recorders and Medicare Systems, India). The absorbance was considered as proportional to living and growing cells.
2.3.4.1 Indirect method. NIH3T3 cells were seeded on the membranes for three and five days. The total protein concentration of the cell-cultured samples was quantified using a bicinchoninic acid (BCA) protein assay.34 Briefly, PBS-rinsed cell-seeded samples were incubated with the BCA working solution (50 parts of BCA reagent with one part of 4% copper sulfate pentahydrate, green colored solution) at 37 °C for 30 min. During the incubation, free amino acids were reduced and formed a crimson-colored complex with BCA. The concentration of this colored complex was assessed from the absorbance at 562 nm on a microplate reader (Recorders and Medicare Systems, India). A standard protein concentration curve was plotted with known concentrations of bovine serum albumin.
2.3.4.2. Direct method. The membranes were incubated in phosphate buffer solution (0.02 M, pH 7.4), containing bovine serum albumin (BSA, 5 g dl−1), human γ-globulin (1.5 g dl−1) and human fibrinogen (0.45 g dl−1), at 37 °C for 2 hours. Subsequently, the samples were gently rinsed with PBS three times. The samples were kept in a 1 wt% aqueous solution of sodium dodecyl sulfate (SDS) for 60 min at room temperature on a shaker. Adsorbed proteins were removed from the samples and measured using a bicinchoninic acid (BCA) protein assay.34
For fluorescence imaging, cell-seeded membranes were washed thrice with PBS and the cells were fixed with 4% paraformaldehyde followed by permeabilization of the cells using cell lysis solution (0.1% Triton-X in PBS). Cells fixed on the films were stained with Hoechst dye (H1399, Invitrogen Life Sciences) according to the manufacturer’s instructions. Images were acquired using an Axio Observer Z1 (Carl Zeiss, Germany).
2.4 Hemocompatibility tests
Hemocompatibility analysis of the prepared membranes is a requisite towards dialysis membrane applications. For the hemocompatibility assays, whole blood was collected from healthy donors in polyethylene disposable syringes containing 4.9% citrate–phosphate–dextrose–adenine (CPDA) solution. The blood was mixed well with anticoagulant solution and the following tests were performed as presented in the subsections. Commercially available fiber as a control was not included or compared in the hemocompatibility studies due to the difficulty in obtaining active (inner) surface blood contact. The sample size was kept similar in each test and each sample was equilibrated with normal saline via incubation for one hour before testing. All the assays were performed in triplicate and their mean values reported.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 volume ratio. Subsequently, 100 μl of this solution was mixed with 600 μl of normal saline. Equal sized-membranes were incubated with the prepared suspension for 1 hour at 37 °C. For the white blood cell (WBC) aggregation study, WBCs were isolated from uncoagulated freshly isolated blood using the Ficoll-Paque mononuclear cell isolation principle with HiSep™ LSM-1077 (Himedia) according to the manufacturer’s instructions. Isolated WBCs were mixed with normal saline and incubated with membranes as previously. After incubation, the cell suspension was smeared on a glass slide and observed under a microscope (Axio Observer Z1Carl Zeiss, Germany).
9 volume ratio. Subsequently, 100 μl of this solution was mixed with 600 μl of normal saline. Equal sized-membranes were incubated with the prepared suspension for 1 hour at 37 °C. For the white blood cell (WBC) aggregation study, WBCs were isolated from uncoagulated freshly isolated blood using the Ficoll-Paque mononuclear cell isolation principle with HiSep™ LSM-1077 (Himedia) according to the manufacturer’s instructions. Isolated WBCs were mixed with normal saline and incubated with membranes as previously. After incubation, the cell suspension was smeared on a glass slide and observed under a microscope (Axio Observer Z1Carl Zeiss, Germany).
 | (6) |
2.5 Urea and creatinine transport
The cast membranes were tested in terms of their urea and creatinine permeances. The set up is described in Fig. 1. Flat sheet membranes, which were cast as described in the previous sections, were cut to fit into the cross flow membrane module. The peristaltic pump drives the feed fluid from the feed tank through the rotameter into the membrane module. The dialysate side fluid is pumped from the dialysate tank by the peristaltic pump through the rotameter into the membrane module. The urea and creatinine permeate through the membrane from the feed to the dialysate side, flowing in a cross flow pattern. The flow rate of the feed side was maintained at 250 ml min−1 and that of the dialysate side was 250 and 500 ml min−1. The concentrations of urea and creatinine in the feed were 500 mg l−1 and 20 mg l−1, respectively.2.6 Statistical analysis
All the experiments were carried out in triplicate. A two-tailed Student’s t-test was carried out for all the data sets and expressed as a mean ± standard deviation (SD). Differences were considered to be significant at p < 0.05. The SD value of each measurement is presented in different figures.3. Results and discussion
3.1 Permeability, MWCO and contact angle
The permeabilities and contact angles for the three membranes are shown in Fig. 2. It is evident from this figure that PAN (S3) is the most hydrophobic membrane with the highest contact angle of 80°. This higher degree of hydrophobicity is linked to depleted hydrogen-bond interaction sites with water. Due to this, hydrophobic solutes experience a spontaneous adsorption onto the membrane,37 resulting in fouling of the membrane surface. However, in the case of dialysis, this problem of protein adsorption leads to bigger complications, such as complement activation. This phenomenon also results in lower flux. The S3 membrane has a permeability of around 0.2 × 10−10 m s−1 Pa, which is the least of the three membranes. The surface-modified membrane (S2) has a higher contact angle than S1, but lower than S3. This can be attributed to the fact that adding PVP to PSf induces hydrophilicity, which reduces the contact angle to 73°. The contact angle of S1 is 69°, the lowest of the three membranes, since it has two hydrophilic polymers in its blend, viz. PVP and PEG. This hydrophilic nature is reflected in the permeability results as well. The permeability of S2 is 0.6 × 10−10 m s−1 Pa, and that of S1 is 1.4 × 10−10 m s−1 Pa. The MWCO of all the membranes is 6 kDa, as presented in Fig. 2(b). | ||
| Fig. 2 (a) Permeabilities and contact angles of the three membranes. (b) MWCO of S1, S2 and S3 membranes. | ||
3.2 Porosity, surface morphology and tensile strength
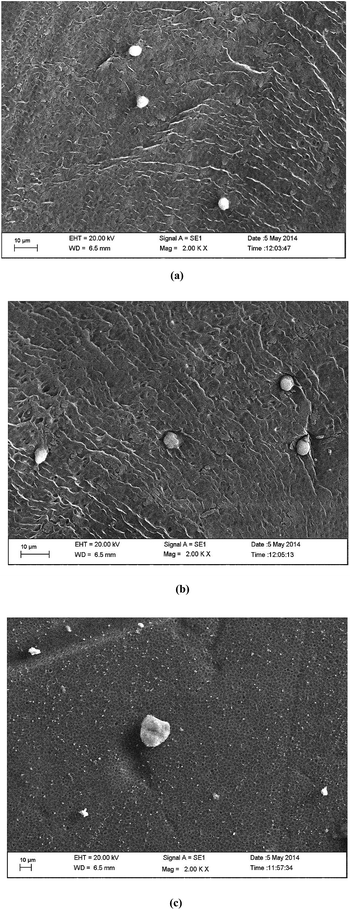
The cross section images show the typical formation of a phase inversion membrane, i.e. a thin skin followed by a porous sub structure, and a spongy bottom layer. The surface morphology can be attributed to the addition of hydrophilic polymers to the blend. During phase inversion, the addition of hydrophilic polymers induces more water flux into the membrane structure, thereby increasing the number of macrovoids. This is reflected in the SEM images (Fig. 3), where S1 has a more porous structure than S2 and S3. However, the skin thicknesses of all three membranes are almost the same and this shows that even though the porous nature of the membranes varies, the MWCO of the membranes being the same, the skin thickness of the membranes is comparable. The porosities and tensile strengths are shown in Fig. 4. It is evident from the above discussion that the porosity of the membranes increases in the order of S3 < S2 < S1. While S3 has a porosity of 54%, S1 has a porosity of 62%. S2 has an intermediate porosity of 60%. The porosity is an exact mirror reflection of the breaking stress relationship. It follows a similar trend, i.e. an increase in the extent of porosity reduces the mechanical strength and thus reduces the breaking stress. Hence, the failure stresses of the S1, S2 and S3 membranes are 6 MPa, 7 MPa and 11 MPa, respectively.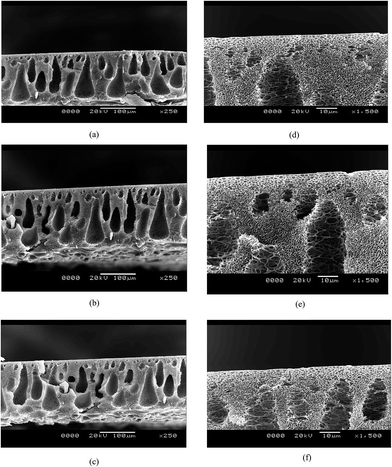 | ||
| Fig. 3 SEM images of the three membranes: (a) cross section of S1; (b) cross section of S2; (c) cross section of S3; (d) skin image of S1; (e) skin image of S2; (e) skin image of S3. | ||
3.3 Surface charge measurement
The surface charges of the three membranes were determined at neutral pH and it was found that S1 and S2 were nearly neutral (0.0 to 0.1 mV). S3 was slightly negative in charge (−0.03 mV), which can be attributed to presence of nitrile groups.3.4 Biological assessments of the membranes
These results further confirmed by the cell proliferation analysis. The cell proliferation activity of the seeded cells was measured by DNA quantification assay as summarized in Fig. 6. This displayed a similar growth pattern to the MTT assay. The cell proliferation rates of all three membranes were significantly higher than the control (39.2 ± 0.1, 55 ± 0.3 on days three and five, respectively). The absorbance value for the S1 seeded cells was 49.4 ± 0.3 on day three of cell seeding followed by 78.08 ± 0.1 on day five; however, the DNA-bound dye absorbance values for seeded cells on S2 and S3 were 41.16 ± 0.7 and 43.59 ± 0.2 on the third day and 60.46 ± 0.3 and 67.15 ± 0.2 on the fifth day, respectively. Here, similar to the metabolic activity, S1 displayed a higher cell proliferation rate, and subsequently higher cell viability, which is expected due to the fact that PSf–PVP membranes are reported to be biocompatible.38 Interestingly, S2, being a surface modified membrane, also exhibited comparable biocompatibility. This fact is further corroborated in the sections discussed below.
According to earlier studies, proteins are preferentially adsorbed on hydrophobic surfaces. In an aqueous system, the initial surface hydration of a hydrophobic material governs the subsequent protein adsorption.9 In this direction, hydrated protein molecules displace the interfacial water through electrostatic interactions to achieve thermodynamic equilibrium.42,43 Therefore, the prepared sampled are supposed to have good protein adsorption properties owing to their nearly hydrophobic nature. Using the direct method, the protein adsorption behavior of the sample membranes incubated with a predefined human plasma protein solution is displayed in Fig. 8. The control sample displayed very high protein absorption (70.41 ± 0.05). S3, being the most hydrophobic membrane (contact angle 80°) among all the prepared membranes, exhibited high protein adsorption. Similar results were obtained for both S2 (contact angle 73°) and S1 (contact angle 69°), having a less hydrophobic nature. S1 displayed the lowest protein adsorption (13.33 ± 0.03) compared to S2 (21.66 ± 0.04) and S3 (32.91 ± 0.05).
Here, it has to be noted that the surface charges, albeit present, are marginal in magnitude. Hence, surface hydrophilicity/hydrophobicity is the dominant factor influencing protein adsorption in such cases.44,45 Hydrophilicity helps in forming a thin layer of aqueous film on the surface of the membrane, impeding the advance and deposition of proteins on the membrane surface, leading to less adsorption of protein. Hence, S1 and S2 experience lower protein adsorption than S3. Moreover, the zwitter-ionic/mixed-charge hydration phenomenon does not exist here owing to the high contact angle (73°) compared to zwitter-ionic surfaces (<20°). During the indirect protein adsorption study, similar results were obtained. S1 displayed the lowest protein adsorption profile compared to S2 and S3.
3.5 Hemocompatibility tests
It has been reported that antifouling properties, surface charge and smoothness control blood cell compatibility and aggregation activity during hemodialysis.46 The promising blood cell compatibility of the S1 membrane is due to the incorporation of PVP, which enhances the hydrophilicity of the membrane, resulting in reduced adsorption of proteins. Further, blending with PSf–PEG causes reduced oxidative stress and limits the surface charge to near neutral, which is also in agreement with reported literature.47 Similar reasons are given for the comparable blood cell compatibility of the S2 membrane. Additionally, phenylenediamine reduces the ROS activity, as discussed previously. S3 also has similar hemolytic activity, but the reduced hydrophilicity and negative surface charge lead to cell aggregation.
The lower platelet adhesion on S1 is believed to be dependent on the protein adsorption behaviour. According to Ishihara et al. (1999), fibrinogen adsorption is prerequisite for platelet adhesion.48 The neutral surface charge results in the repulsion of proteins and negatively charged platelets. Tanaka et al. (2000) observed that besides fibrinogen adsorption, its conformation also needs to be changed for platelet adhesion.49 Adsorbed fibrinogen in native conformation never participates in platelet adhesion and activation. In this context, the S2 sample has lower fibrinogen adsorption without significant conformation changes compared to S3, resulting in low platelet adhesion and no aggregation.
3.6 Urea and creatinine permeation
The urea and creatinine transport through the membranes are shown in Fig. 14. It is evident from the figure that S1 has a higher uremic toxin transport rate than S2 or S3. While the time taken for S1 and S2 to bring down the urea concentration in the feed tank to the desired level (400 mg l−1) is around 120 minutes, the same for S3 is 180 minutes. Similarly, creatinine transport requires 120 minutes for S1 to bring down the concentration to 12 mg l−1. However, the rates are much slower for S2 and S3. This can be explained on the basis of the permeability behaviour of the three membranes (Section 3.1). While the permeability of S1 is the highest, that of S3 is lowest. Given that the membranes have the same MWCO, this would automatically imply that the transport rate of the solutes would decrease as permeability decreases. | ||
| Fig. 14 Uremic toxin transport through the three membranes: (a) urea transport, (b) creatinine transport. | ||
3.7 Comparison with state of the art literature
Tables 1 and 2 illustrate a detailed comparison between the present work and recently reported literature. It is evident from Table 1 that detailed membrane characterization has been carried out in the present work. Hydraulic permeability, MWCO and surface hydrophilicity have been investigated and the reported values are comparable with the literature data. However, mechanical strength, porosity and surface charge are a few of the additional aspects that have been quantified in the present work. The important interplay of these characteristics with biocompatibility has already been discussed in earlier sections. Table 2 presents a detailed comparison of the present work in terms of cytocompatibility and hemocompatibility with the available literature. Two important aspects are evident from this information. First, exhaustive cytocompatibility and hemocompatibility tests have been carried out in this work totalling 9 parameters as described in Table 2. Second, the developed materials in the present work show competitive results with the reported literature.| Membrane properties | Present work | State of art literature15–20,36 |
|---|---|---|
| Hydraulic permeability (m s−1 Pa) | 0.2–1.4 × 10−10 | 3.12 × 10−11 to 4.16 × 10−10 |
| Molecular weight cut off (kDa) | 6 | — |
| Contact angle | 68°–80° | 56°–72° |
| Tensile strength (MPa) | 6–11 | — |
| Porosity (%) | 53–62 | — |
| Surface charge (mV) | −0.03–0.1 | — |
| Surface morphology (SEM) | Studied | Studied |
| Material | Present work | State of art literature | |||||
|---|---|---|---|---|---|---|---|
| Li et al.15 | Nie et al.16 | Higuchi et al.17 | Dahe et al.18 | Shakaib et al.19,20 | Lin et al.36 | ||
| PSf–PVP–PEG; surface modified PSf; PAN homopolymer | PES blended with citric acid-grafted polyurethane | Carbon nanotube-grafted PES composite | Chemically modified PSf | PSf–vitamin E–TPGS composite | Polyamide and monosodium glutamate blend | PAN immobilized with chitosan and heparin | |
| Cytocompatibility | |||||||
| Metabolic activity (% better than control) | √√ (37%) | √√ (24%) | √√ (30%) | — | √√ (33%) | — | |
| Cell proliferation | √√ | — | — | — | √√ | — | — |
| Oxidative stress | √√ | — | — | — | √√ | — | — |
| Total protein adsorption (μg cm−2) | √√ 13–32 | √√ 18–34 | √√ 7–15 | √√ 3–8 | √√ 18–30 | — | — |
| Cell attachment and morphology (SEM and confocal imaging) | √√ | — | — | — | √√ | — | — |
| Hemocompatibility | |||||||
| Hemolysis | √√ | — | — | — | √√ | — | — |
| Blood cell aggregation | √√ | — | — | — | — | — | — |
| Platelet adhesion | √√ | √√ | √√ | √√ | √√ | — | — |
| Thrombus formation | √√ | — | — | — | √√ | — | — |
4. Conclusions
(i) Three types of dialysis grade membrane of identical MWCO were synthesized and characterized in detail. The permeability, mechanical strength, contact angle, surface charge, porosity and membrane morphology were analyzed. It was found that the S1 (PSf–PVP–PEG) blend membrane had the highest permeability (1.2 × 10−10 m Pa−1 s), smallest contact angle (69°) and highest porosity (62%). The surface charge of the membrane was found to be near neutral.(ii) The biological assessment of the three membranes was carried out comprehensively. It was found that S1, S2 and S3 were cytocompatible, displaying promising proliferation and metabolic activity as well as minimal cell adhesion and ROS activity.
(iii) The hemocompatibility performances of the synthesized membranes were comparable. The blood cell aggregation activities were similar in all three membranes. Interestingly, the prepared membranes exhibited very low hemolysis activity compared to the control. Further, a varying trend of platelet adhesion was found among the three membranes owing to their protein adhesion nature. S1 adhered less platelets compared to S2 and S3, which followed in the thrombus formation activity. Overall S1 and S2 have comparable hemocompatibilities and are better than S3 and the commercial membrane.
(iv) The transport of uremic toxins indicated that S1 and S2 facilitated the permeation more than S3. While it took only 120 minutes for S1 and S2 to bring down the urea concentration from 500 to less than 400 mg l−1, the same for S3 was 180 minutes. Similar results were also obtained for creatinine transport.
Acknowledgements
This work is partially supported by a grant from the Department of Science and Technology, Government of India, and Forus under the scheme no. IDP/MED/7/2011, dt. 05.03.2012. Any opinions, findings and conclusions expressed in this paper are those of the authors and do not necessarily reflect the views of the DST.References
- The Renal Association, Normal GFR, 2013, http://www.renal.org/information-resources/the-uk-eckd-guide/normal-gfr#sthash.Qoxv8mmG.dpbs.
- W. J. Kolff and H. T. J. Berk, Acta Med. Scand., 1944, 117, 121–134 CrossRef PubMed.
- N. Alwall, Therapeutic and Diagnostic Problems in Severe Renal Failure, Munksgaard, Copenhagen, 1963 Search PubMed.
- T. Graham, Philos. Trans. R. Soc. London, 1854, 144, 177–228 CrossRef.
- A. Fick, Ann. Phys. Chem., 1855, 94, 59–86 CrossRef.
- W. R. Clark, R. J. Hamburger and M. J. Lysaght, Kidney Int., 1999, 56, 2005–2015 CrossRef CAS PubMed.
- T. Kinoshita, Immunol. Today, 1991, 12, 291–294 CrossRef CAS.
- S. Bowry and T. Rintellen, ASAIO J., 1998, 44, 579–583 CrossRef PubMed.
- M. C. Yang and W. C. Lin, J. Polym. Res., 2002, 9, 201–206 CrossRef CAS.
- H. G. Hicke, P. Bohme, M. Becker, H. Schulze and M. Ulbricht, J. Appl. Polym. Sci., 1996, 80, 1147–1161 CrossRef.
- M. Ulbricht and A. Papra, Enzyme Microb. Technol., 1997, 20, 61–68 CrossRef CAS.
- M. Ulbricht and G. Belfort, J. Membr. Sci., 1996, 111, 193–215 CrossRef CAS.
- M. Hayama, T. Miyasaki, S. Mochizuki, H. Asahara, K. Tsujioka, F. Kohori, K. Sakai, Y. Jinbo and M. Yoshida, J. Membr. Sci., 2002, 210, 45–53 CrossRef CAS.
- A. C. Yamashita, Contrib Nephrol, Karger, Basel, 2011, vol. 173, pp. 58–69 Search PubMed.
- L. Li, C. Cheng, T. Xiang, M. Tang, W. Zhao, S. Shun and C. Zhao, J. Membr. Sci., 2012, 405–406, 261–274 CrossRef CAS PubMed.
- C. Nie, L. Ma, Y. Xia, C. He, J. Deng, L. Wang, C. Cheng, S. Sun and C. Zhao, J. Membr. Sci., 2015, 475, 455–468 CrossRef CAS PubMed.
- A. Higuchi, K. Shirano, M. Harashima, B. O. Yoon, M. Hara, M. Hattori and K. Imamura, Biomaterials, 2002, 23, 2659–2666 CrossRef CAS.
- G. J. Dahe, R. S. Teotia, S. S. Kadam and J. Bellare, Biomaterials, 2011, 32, 352–365 CrossRef CAS PubMed.
- M. Shakaib, I. Ahmed, R. M. Yunus and A. Idris, Int. J. Polym. Mater. Polym. Biomater., 2013, 62, 345–350 CrossRef CAS.
- M. Shakaib, I. Ahmed, R. M. Yunus and M. Z. Noor, Int. J. Polym. Mater. Polym. Biomater., 2014, 63, 80–85 CrossRef.
- A. Saito, Contrib Nephrol, Karger, Basel, 2011, 173, pp. 1–10 Search PubMed.
- S. K. Bowry, E. Gatti and J. Vienken, Contrib Nephrol., Karger, Basel, 2011, vol. 173, pp. 110–118 Search PubMed.
- A. M. MacLeod, M. K. Campbell, J. D. Cody, C. Daly, A. Grant, I. Khan, K. S. Rabindranath, L. Vale and S. A. Wallace, Cochrane Database of Systematic Reviews, 2005, issue 3, CD003234 Search PubMed.
- S. K. Bowry, Int. J. Artif. Organs, 2002, 25, 447–460 CAS.
- S. Qiu, L. Wu, L. Zhang, H. Chen and C. Gao, J. Appl. Polym. Sci., 2009, 112, 2066–2072 CrossRef CAS.
- A. Roy and S. De, J. Food Eng., 2014, 126, 7–16 CrossRef CAS PubMed.
- P. Rai, G. C. Majumdar, S. Dasgupta and S. De, LWT–Food Sci. Technol., 2007, 40, 1765–1773 CrossRef CAS PubMed.
- S. Banerjee and S. De, J. Membr. Sci., 2012, 389, 188–196 CrossRef CAS PubMed.
- J. F. Li, Z. L. Xu, H. Yang, L. Y. Yu and M. Liu, Appl. Surf. Sci., 2009, 255, 4725–4732 CrossRef CAS PubMed.
- S. R. Panda and S. De, J. Polym. Res., 2013, 20, 179–195 CrossRef PubMed.
- S. Chatterjee and S. De, Sep. Purif. Technol., 2014, 125, 223–238 CrossRef CAS PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- B. Das, P. Dadhich, P. Pal, P. K. Srivas, K. Bankoti and S. Dhara, J. Mater. Chem. B, 2014, 2(39), 6839–6847 RSC.
- P. K. Smith, R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson and D. C. Klenk, Anal. Biochem., 1985, 85, 76–85 CrossRef.
- F. Pati, P. Datta, B. Adhikari, S. Dhara, K. Ghosh and P. K. Das Mohapatra, J. Biomed. Mater. Res., Part A, 2012, 100(4), 1068–1079 CrossRef PubMed.
- W.-C. Lin, T.-Y. Liu and M.-C. Yang, Biomaterials, 2004, 25, 1947–1957 CrossRef CAS PubMed.
- W. Sun, J. Liu, H. Chu and B. Dong, Membranes, 2013, 3, 226–241 CrossRef CAS PubMed.
- M. Hayama, K. Yamamoto, F. Kohori and K. Sakai, J. Membr. Sci., 2004, 234, 41–49 CrossRef CAS PubMed.
- M. Matsumoto, M. Yamaguchi, Y. Yoshida, M. Senuma, H. Takashima, T. Kawamura and A. Hirose, Food Chem. Toxicol., 2013, 56, 290–296 CrossRef CAS PubMed.
- Chemicalland21, 2012, N,N′-Diphenyl-p-Phenylenediamine.
- T. Satoh and M. Izumi, Neurosci. Lett., 2007, 418(1), 102–105 CrossRef CAS PubMed.
- M. M. Santore and C. F. Wertz, Langmuir, 2005, 21, 10172–10178 CrossRef CAS PubMed.
- K. Nakanishi, T. Sakiyama and K. Imamura, J. Biosci. Bioeng., 2001, 91(3), 233–244 CrossRef CAS.
- T. Godjevargova and A. Dimov, J. Membr. Sci., 1992, 67, 283–287 CrossRef.
- S. Y. Wong, L. Han, K. Timachova, J. Veselinovic and N. Hyder, Biomacromolecules, 2012, 13(3), 719–726 CrossRef CAS PubMed.
- Z.-G. Wang, L.-S. Wan and Z.-K. Xu, J. Membr. Sci., 2007, 304, 8–23 CrossRef CAS PubMed.
- C. R. Hasler, G. R. Owen, W. Brunner and W. H. Reinhart, Nephrol., Dial., Transplant., 1998, 13, 3132–3137 CrossRef CAS PubMed.
- K. Ishihara, K. Fukumoto, Y. Iwasaki and N. Nakabayashi, Biomaterials, 1999, 20, 1553–1559 CrossRef CAS.
- M. Tanaka, T. Motomura, M. Kawada, T. Anzai, K. Yuu, T. Shiroya, K. Shimura, M. Onishi and A. Mochizuki, Biomaterials, 2000, 21, 1471–1481 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |