Synthesizing graphenes directly on SiO2/Si in open environments by a dual flame method
Abstract
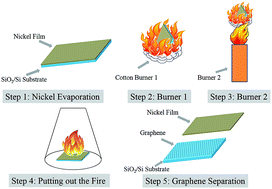
In this work, a simple, productive and low cost method is reported for synthesizing few-layer graphenes directly on SiO2/Si substrates. Films of nickel with different thicknesses (25–700 nm) are thermally deposited on SiO2/Si substrates as catalyst. The substrates with nickel films are treated by lighting cotton and alcohol blast burners in sequence and then cooling down quickly. The dual flames last only several minutes. After growth, few-layer graphenes can be found on the upper surface of nickel and at the interfaces between the nickel and the SiO2/Si, which are confirmed by Raman spectroscopy. Few-layer graphenes can be directly obtained on the SiO2/Si substrate after etching off nickel, without further transfer process. Scanning Raman mapping and transmission electron microscopy indicate that the graphenes are uniform, continuous and of high quality. The dependence of the quality of graphenes on the thickness of nickel have been studied and discussed.

 Please wait while we load your content...
Please wait while we load your content...