Interaction between red wine procyanidins and salivary proteins: effect of stomach digestion on the resulting complexes
S. Soares*,
E. Brandão,
N. Mateus and
V. de Freitas
REQUIMTE\LAQV, Departmento de Química e Bioquímica, Faculdade de Ciências, Universidade do Porto, Rua do Campo Alegre, 689, 4169-007 Porto, Portugal. E-mail: susana.soares@fc.up.pt
First published on 13th January 2015
Abstract
Tannins, a group of polyphenols, are important at sensory (e.g. astringency sensation) and health levels (e.g., anti-cancer and cardiovascular protection). The health benefits are related to tannins' concentration that reaches the gastrointestinal tract (bioaccessible concentration) which could be affected by interaction with other biological molecules, such as salivary proteins (SP). Most of the work that studies tannin's health benefits does not consider these interactions. So, this work intended to mimic the ingestion of red wine condensed tannin fractions and assess the stability of the (in)soluble complexes formed between the different tannins and the different SP in a simulated stomach digestion. The results showed that some of the tannin/SP complexes could be disrupted by gastric digestion leading to the release of tannins. This was observed for the complexes formed with the lowest polymerized tannins (monomers, dimers and trimers). Oppositely, the complexes formed by tannin tetramers and pentamers were significantly more resistant to stomach conditions. Therefore, SP probably influence negatively the concentrations at which tannins tetramers and pentamers reach the stomach and ultimately they may influence negatively some of these procyanidins biological potential health benefits. In the future, these and other biological interactions of tannin compounds should be taken into consideration in bioavailability and health benefits studies.
Introduction
Tannins are a group of polyphenol compounds that are widely distributed in vegetal foodstuffs, particularly in fruits, cereal grains and derived beverages (e.g. red wine, tea and beer).1The designation “tannin” has its origin in the use of wood tannins from oak in the tanning process of animal hides into leather. Presently, tannins comprise a complex group of water-soluble phenolic compounds with a huge range of molecular weights (0.5 to around 20 kDa)2 that have the special ability of interacting with proteins, eventually leading to their precipitation. These compounds are structurally divided in two major classes, condensed tannins (polymers of flavan-3-ol units) and hydrolysable tannins (esters of glucose with gallic acid).
Tannins are important at both sensory and health levels. Regarding organoleptic properties, tannins are directly related to astringency sensation and contribute also to bitter taste. Astringency is a tactile sensation being described as dryness, tightening and puckering sensations3 perceived in the oral cavity during the ingestion of foodstuffs rich in tannins. Several mechanisms have been proposed to its origin, however the most accepted by the scientific community arises from the interaction between tannins and salivary proteins (SP) leading to their precipitation.4 In fact, during foodstuff consumption, tannins interact with SP, especially with proline-rich proteins (PRPs) forming (in)soluble aggregates. In general, the nature of tannin/protein interactions can be described as covalent or non-covalent based on whether the molecules are irreversibly bound to each other or not, and which could result in the formation of soluble or insoluble complexes. The interactions are thought to involve the cross-linking of separate protein molecules by the tannin which acts as a polydentate ligand on the protein surface involving hydrophobic and hydrogen bonds.
Regarding the SP, the main proteins have been grouped into six structurally related major classes: histatins, basic PRPs (bPRPs), acidic PRPs (aPRPs), glycosylated PRPs (gPRPs), statherin, and cystatins.5,6 The differences between the several families of PRPs depend on their charge and presence or absence of carbohydrates. All these proteins have important biological functions in saliva including calcium binding to enamel, maintenance of ionic calcium concentration, antimicrobial action or protection of oral tissues against degradation by proteolytic activity.7–12
Additionally to the sensory properties, tannins and polyphenols in general have received high attention in the past years due to the several important health benefits associated to their ingestion. Several epidemiological studies have associated these compounds to benefic actions such as anti-cancer, anti-neurodegenerative activities, cardiovascular protection.13,14 One classical association is the well-known “French-paradox”.15 The French population showed a low incidence of cardiovascular diseases despite the high consumption of saturated fat and tabaco. This fact was attributed to a regular consumption of red wine. In fact, red wine is one of the most rich and highly consumed source of tannins worldwide.16,17
One key aspect to study the health effect of a specific compound is to determine the amount that reaches the gastrointestinal tract (herein referred as bioaccesible concentration) and, subsequently, the target tissue/organ (included in a more wide term, bioavailable concentration). In the case of tannins, there is only scarce quantity of data on these aspects because there are some technical increased limitations on tannin analysis (equipment sensibility, lack of standard molecules, complex polymers difficult to extract, isolate and analyze). Besides these limitations, the determination of tannins bioaccessible concentration is even more complex because some compounds have important interactions with other biological compounds, namely SP, as referred previously. In fact, most of the works that study the health benefits of these compounds do not take into account these interactions.
In this way, one important consideration for the bioaccessibility of tannins, it is their ability to interact forming (in)soluble complexes with SP present in human saliva and the stability of these complexes in stomach conditions. This can modify the accessibility of tannins in the gastrointestinal tract.
So, this work intended to mimic the ingestion of condensed tannin fractions with increasing degrees of polymerization prepared in a wine model solution (since red wine is a natural, highly consumed and rich source of procyanidins) and assess the stability of the insoluble complexes eventually formed between the different tannins and the different classes of SP in a simulated stomach digestion mode.
Results and discussion
In the oral cavity, tannins or procyanidins have the characteristic property to interact with SP forming (in)soluble complexes and leading to astringency sensation, as already referred. However, there is not much knowledge on how this interaction affects tannins accessibility (bioaccessibility) in the gastric system. So, in this work it was intended to simulate the ingestion of red wine procyanidins to study the stability of the eventually formed complexes (procyanidins/SP) in a simulated gastric digestion environment.Grape seed fraction (GSF) characterization
GSF where characterized by reaction with phloroglucinol in order to determine the mDP of each fraction. Based on previous works it is expected that the polymerization degree increases with the fractions obtained.18 Fraction 1 was found to contain mainly catechins and gallic acid but also a small quantity of procyanidin dimers (mean DP 1.1). F2 contains essentially catechins and galloyl derivatives as well as procyanidin dimers and galloyl derivatives (mean DP 1.4). F3 contains mainly procyanidin dimers and trimers and their galloyl derivatives but also a small quantity of procyanidin tetramers (mean DP 2). F4 contains mainly procyanidin trimers and tetramers, their galloyl derivatives and also procyanidin pentamers (mean DP 4). F5 contains mainly procyanidin tetramers and pentamers and their galloyl derivatives but also hexamers galloylated (mean DP 5).SP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) procyanidins ratio and interaction between SP and procyanidins
procyanidins ratio and interaction between SP and procyanidins
The SP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) procyanidins ratio was chosen according to the literature19 and considering that a volume of 10 mL is usually used when simulating the drinking process.
procyanidins ratio was chosen according to the literature19 and considering that a volume of 10 mL is usually used when simulating the drinking process.
Regarding the saliva and in order to make the in vitro interaction similar to what happens in the oral cavity, it was considered that saliva is dynamic being continuously produced during the tasting of wine. The volume of saliva normally present in mouth (residual saliva) is around 0.75 mL and the continuous flow of further saliva secreted by the salivary glands in responsive to wine is known to be 1 mL min−1. According to sensory protocols,20 when wine is introduced in the mouth, the maximum astringency is reached only after 15 s, thus the volume of saliva produced within 15 s resulted to be 0.25 mL. The total salivary volume that comes in contact with wine and produced within 15 s resulted in 1 mL (0.75 + 0.25 mL) or 4 mL min−1. However, some in vitro studies and also some sensory studies usually use 5 min to study this interaction and then the volume of saliva in this time becomes 20 mL. Since 10 mL of wine is exposed to 20 mL (4 mL min−1 × 5 min) of saliva for 5 min, a saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wine ratio of 2
wine ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 seems closer to reality.19
1 seems closer to reality.19
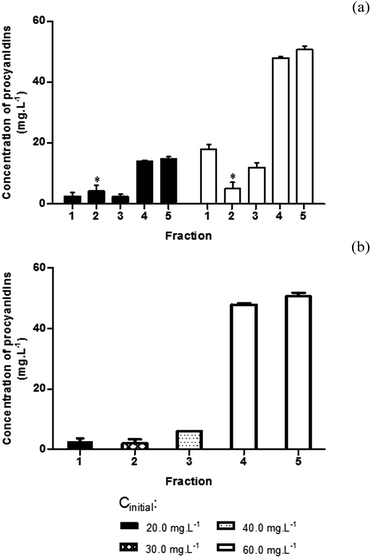
In order to compare the ability of the different GSF to interact and precipitate the different families of SP, all fractions reacted with saliva at the minimum and maximum concentrations studied (20.0 mg L−1 and 60.0 mg L−1, respectively). After the interaction, the precipitates were removed by centrifugation and the procyanidins that remain in the supernatant were determined by reaction with 3-[4-(dimethylamino)phenyl]prop-2-enal (DMACA). The procyanidins concentration present in the precipitate was calculated by subtracting the supernatant concentration value to the initial procyanidins concentration (Fig. 1).
From the results presented in Fig. 1(a) it is possible to observe that for both (minimum and maximum) concentrations, the most reactive fractions toward SP precipitation were F4 and F5. This means that the most polymerized fractions are the most effective ones in the interaction/precipitation of SP. In fact, it is possible to observe that for the same concentration increasing the mDP (F1 < F2 < F3 < F4 < F5) increases interaction with SP. This fact is also visible in the results for the other tested concentrations (30.0 and 40.0 mg L−1 for F2 and F3, respectively) (Fig. 1(b)). The only unexpected result it was that obtained with the F1 at 60.0 mg L−1. The fact that tannins interaction with proteins increases either with concentration, polymerization degree and galloylation has been well supported in the literature.21–23
After comparing the reactivity of the different GSF towards SP, the following experiments were done only for the concentrations that were reported to exist in red wine taking into consideration the mDP: F1, 20.0 mg L−1; F2, 30.0 mg L−1; F3, 40.0 mg L−1; F4 and F5, 60.0 mg L−1.24
The protein profile of the supernatants was analyzed by HPLC before (control) and after the interaction with GSF in order to determine which SP reacted and were precipitated by procyanidins (Fig. 2(a)).
The HPLC chromatogram of the supernatant control solution at 214 nm is presented in Fig. 2. The top of the figure shows the distribution of the different families of SP along the chromatogram that were established previously by proteomic approaches, namely ESI-MS and MALDI-TOF/TOF.21
The HPLC chromatogram of the control solution is roughly divided into four SP family regions: the first region comprises proteins that belong to the classes of bPRPs and histatins. The bPRPs identified in this region include IB-8b, IB-8c, IB-9, IB-4 and P-J and the histatins include histatins 3, 5, 7, 8 and 9. The second region comprises mainly one gPRPs, the bPRP3. The next region corresponds entirely to aPRPs, namely PRP1 and PRP3, and the last region has phosphorylated and non-phosphorylated forms of statherin and peptide P-B.
As an example of the observed changes in SP HPLC profile, the results for the interaction with 60.0 mg L−1 of F5 are presented in Fig. 2(a). The observed changes of the chromatographic peaks area of the different SP with the different GSF were expressed in percentage of the area of these proteins relatively to the respective area in the control saliva (Fig. 2(b)).
From the presented results it is possible to observe that the most precipitated proteins were statherin and aPRP, in particular for F3, F4 and F5. bPRP are not affected for any GSF even for the most polymerized ones while gPRPs start to be precipitated for the most polymerized fractions. Probably it would be necessary a higher concentration of GSF to precipitate these proteins, as observed in previous works.21,25
The analyses of the SP are in accordance with the quantification of GSF in the supernatant for the correspondent concentration. For instance, F4 and F5 at 60.0 mg L−1 were the most reactive and most precipitated GSF (Fig. 1(b)) and also in the HPLC SP profile where they were the ones that depleted almost completely statherin and reduced significantly aPRPs. However, F5 also starts to reduce gPRP (∼20%). Oppositely, F1 and F2 at 20.0 and 30.0 mg L−1, respectively, showed a very small interaction with SP by GSF quantification (Fig. 1(b)) and in the HPLC profile analysis of SP (Fig. 2(b)).
MALDI-TOF analysis of the precipitates
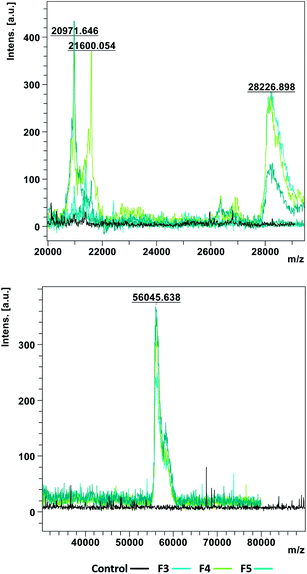
In order to obtain information and characterize the insoluble complexes formed by interaction of SP with F3, F4 and F5, the precipitates were resuspended or resolubilized for MALDI-TOF analysis. In this experiment some difficulties were observed: the total resolubilization was extremely difficult and in the case of F4 and F5 it was only possible to do a parcial resolubilization and the resuspension of precipitates was not homogenous. These difficulties suggest that the insoluble complexes formed are extremely stable and so probably they are originated by covalent bonds with tannins and proteins being irreversibly bound to each other.Nevertheless, the complexes that were resuspended or resolubilized were analyzed and the results are presented in Fig. 3.
The results showed clearly the appearance of new and high molecular weight peaks that were not present in the control condition (saliva and model wine solution). In Fig. 3 it is possible to observe the appearance of peaks with small masses around 20 and 28 kDa but also with higher masses around 60 kDa.
As referred herein and in previous work,21 most of the SP analyzed by the described methodology are indeed peptides with small molecular weights and with different isoforms and post-translational modification, such as phosphorylation. Statherin have a molecular weight around 5 kDa, aPRP have a molecular weight around 11–15 kDa, histatins have a molecular weight around 3 kDa and bPRP have a molecular weight around 4–6 kDa. As the identified masses are significantly higher than these values, this analysis supports that SP and tannins interact forming a huge network of protein/tannin complexes with very high molecular weights.
However, it is important to refer that the analysis of the precipitates is very difficult and the solutions were not homogenous. So, it is extremely difficult to obtain information about the molecular composition of these precipitates.
Stability of tannins/SP complexes to gastric digestion
After comparing the reactivity of the different GSF toward SP and determining which SP were precipitated by the different GSF, it was intended to study the stability of the formed tannin/SP complexes during stomach digestion. So, the precipitates were incubated for 2 h in a solution simulating stomach conditions with continuous shaking. After this incubation the procyanidins eventually released to the solution (supernatant) were quantified by reaction with DMACA. The obtained results are presented in Fig. 4.From the results presented in Fig. 4 it is possible to observe that for F1, F2 and F3 the concentration of procyanidins in the precipitates (before) and supernatant (after) digestion are not-significantly different. Therefore, it seems that for the lowest polymerized fractions the complexes formed with SP could be disrupted by the stomach environment leading to the release of procyanidins to the supernatant. On the other hand, the results for F4 and F5 are quite different. From Fig. 4 it is possible to observe that after digestion the concentration of procyanidins is approximately half or less of the concentration initially present in the precipitates. This means that the complexes formed by these fractions with SP are more resistant to the digestion conditions.
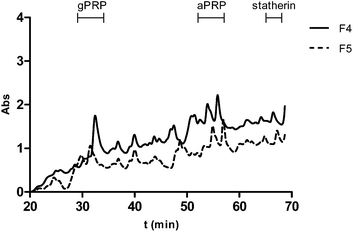
The supernatants after the simulated digestion of the precipitates of the most reactive fractions (F4 and F5) were also analyzed by HPLC in order to determine which SP were released from the complexes. The results are presented in Fig. 5.
From the results presented in Fig. 5 it is possible to observe that the major SP that are released from the complexes are gPRP and aPRP. In fact, statherin it seems to be released in a very small extent. Somehow the complexes statherin/procyanidins are more stable and resistant than the ones formed with proline-rich proteins family.
These results have important consequences at the biological level. Although the lowest polymerized procyanidins (F1, F2 and F3) are the ones that are present in red wine in lowest concentrations, they seem to be the ones more readily bioaccessible. They have the lowest interaction with SP and when they interact with SP the eventually formed insoluble complexes are disrupted by the gastric digestion. So, overall, the monomeric and dimeric procyanidins probably reach the gastrointestinal environment in small protein/procyanidins complexes or intact at the concentrations that exist in red wine and could display beneficial biological activities such as anticancer activity26 or cardiovascular protection but also harmful actions such as inhibition of digestive enzymes.27
Regarding the most polymerized fractions (F4 and F5), at the concentration present in red wine they are practically depleted by SP and they reach the gastrointestinal environment in the form of insoluble complexes. As the results showed some of these complexes could be disrupted by the gastric digestion releasing these procyanidins to exert some biological activities or they could be catabolized into small phenolic molecules by the intestinal microflora.28
Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v/v). The resulting solution was centrifuged and the chloroform phase, containing chlorophylls, lipids and other undesirable compounds was rejected. The hydroalcoholic phase was then extracted with ethyl acetate, and the organic phase was evaporated using a rotary evaporator (30 °C). The resulting residue corresponding essentially to oligomeric procyanidins was fractionated through a TSK Toyopearl HW-40(s) gel column (100 mm × 10 mm i.d., with 0.8 mL min−1 methanol as eluent), yielding five fractions according to the method described in the literature.29 The first 30 min of elution were rejected. The first (F1), second (F2) and third (F3) fractions were obtained after elution with 99.8% (v/v) methanol during 15 min (12 mL), other 15 min (12 mL) and other 4 h (192 mL), respectively. The fourth fraction (F4) was eluted with methanol/5% (v/v) acetic acid during the next 14 h (670 mL) and the fifth fraction (F5) was eluted with methanol/10% (v/v) acetic acid during the next 8 h (384 mL). All fractions were mixed with deionized water, and the organic solvent was eliminated using a rotary evaporator under reduced pressure at 30 °C and then freeze-dried.
2, v/v/v). The resulting solution was centrifuged and the chloroform phase, containing chlorophylls, lipids and other undesirable compounds was rejected. The hydroalcoholic phase was then extracted with ethyl acetate, and the organic phase was evaporated using a rotary evaporator (30 °C). The resulting residue corresponding essentially to oligomeric procyanidins was fractionated through a TSK Toyopearl HW-40(s) gel column (100 mm × 10 mm i.d., with 0.8 mL min−1 methanol as eluent), yielding five fractions according to the method described in the literature.29 The first 30 min of elution were rejected. The first (F1), second (F2) and third (F3) fractions were obtained after elution with 99.8% (v/v) methanol during 15 min (12 mL), other 15 min (12 mL) and other 4 h (192 mL), respectively. The fourth fraction (F4) was eluted with methanol/5% (v/v) acetic acid during the next 14 h (670 mL) and the fifth fraction (F5) was eluted with methanol/10% (v/v) acetic acid during the next 8 h (384 mL). All fractions were mixed with deionized water, and the organic solvent was eliminated using a rotary evaporator under reduced pressure at 30 °C and then freeze-dried.The control condition was a mixture of S (200 μL) and model solution (100 μL) (final volume 300 μL). Different volumes of GSF stock solutions (400 mg L−1) prepared in model solution were added to S (200 μL) to obtain the desired final concentrations. The final volume was adjusted to 300 μL with model solution. The ratio used (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 saliva
1 saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wine) has been used previously as a model closer to reality of wine ingestion.19 The mixture was shaken and kept for 5 min at room temperature (±20 °C) and then centrifuged (8000g, 5 min). The supernatant was separated from the precipitate. Part of the supernatant was injected into the HPLC to monitor the SP present and other part was analyzed by spectrometry after reaction with DMACA to measure the procyanidin content. The precipitate was subjected to simulated stomach/gastric digestion (Fig. 6).
wine) has been used previously as a model closer to reality of wine ingestion.19 The mixture was shaken and kept for 5 min at room temperature (±20 °C) and then centrifuged (8000g, 5 min). The supernatant was separated from the precipitate. Part of the supernatant was injected into the HPLC to monitor the SP present and other part was analyzed by spectrometry after reaction with DMACA to measure the procyanidin content. The precipitate was subjected to simulated stomach/gastric digestion (Fig. 6).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a matrix solution (3 mg mL−1) of α-cyano-4-hydroxycinnamic acid matrix prepared in 50% methanol. Aliquots of samples (2 μL) were spotted onto the MALDI sample target plate, and spectra were obtained in the mass range between 1500 and 200
1) with a matrix solution (3 mg mL−1) of α-cyano-4-hydroxycinnamic acid matrix prepared in 50% methanol. Aliquots of samples (2 μL) were spotted onto the MALDI sample target plate, and spectra were obtained in the mass range between 1500 and 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da with ca. 2000 laser shots.
000 Da with ca. 2000 laser shots.Conclusions
This work provided evidences that SP probably influence negatively the concentration at which tetramers and pentamers of procyanidins reach the stomach due to their precipitation and ultimately they may influence negatively some of these procyanidins biological potential health benefits. This effect was opposite for procyanidin monomers, dimers and trimmers which reach the gastrointestinal environment at the concentrations they exist in red wine or in small protein/procyanidins complexes that are disrupted by stomach digestion.These results also support the hypothesis that the high and resistant ability of some SP to precipitate the most polymerized tannins could be related to the prevention of deleterious effects of tannin compounds in the digestive tract (e.g., inhibition of digestive enzymes).
Acknowledgements
The authors thank the financial support by one postdoctoral fellowship (SFRH/BPD/88866/2012) from FCT (Fundação para a Ciência e Tecnologia). The authors thank Dra Silvia Maia and Dra Rosa for MALDI-TOF analysis.References
- A. Scalbert and G. Williamson, J. Nutr., 2000, 130, 2073S–2085S CAS.
- J. M. Souquet, V. Cheynier, F. Brossaud and M. Moutounet, Phytochemistry, 1996, 43, 509–512 CrossRef CAS.
- ASTM, Standard Terminology to Sensory Evaluation of Materials and Products, American Society of Testing and Materials, Philadelphia, PA, 1989 Search PubMed.
- V. de Freitas and N. Mateus, Curr. Org. Chem., 2012, 16, 724–746 CrossRef CAS.
- N. Huq, K. Cross, M. Ung, H. Myroforidis, P. Veith, D. Chen, D. Stanton, H. He, B. Ward and E. Reynolds, Int. J. Pept. Res. Ther., 2007, 13, 547–564 CrossRef CAS.
- S. P. Humphrey and R. T. Williamson, J. Prosthet. Dent., 2001, 85, 162–169 CrossRef CAS PubMed.
- D. I. Hay, A. Bennick, D. H. Schlesinger, K. Minaguchi, G. Madapallimattam and S. K. Schluckebier, Biochem. J., 1988, 255, 15–21 CAS.
- F. G. Oppenheim, T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, G. D. Offner and R. F. Troxler, J. Biol. Chem., 1988, 263, 7472–7477 CAS.
- D. I. Hay, D. J. Smith, S. K. Schluckebier and E. C. Moreno, J. Dent. Res., 1984, 63, 857–863 CrossRef CAS PubMed.
- D. L. Kauffman and P. J. Keller, Arch. Oral Biol., 1979, 24, 249–256 CrossRef CAS.
- D. L. Kauffman, P. J. Keller, A. Bennick and M. Blum, Crit. Rev. Oral Biol. Med., 1993, 4, 287–292 CAS.
- E. J. Helmerhorst and F. G. Oppenheim, J. Dent. Res., 2007, 86, 680–693 CrossRef CAS PubMed.
- I. C. Arts and P. C. Hollman, Am. J. Clin. Nutr., 2005, 81, 317S–325S CAS.
- A. Valavanidis and T. Vlachogianni, in Studies in Natural Products Cemistry, ed. Atta-ur-Rahman, Elsevier, Amsterdam, 2013, vol. 39, pp. 9–10 Search PubMed.
- S. Renaud and M. de Lorgeril, Lancet, 1992, 339, 1523–1526 CrossRef CAS.
- Y. Wang, S.-J. Chung, W. O. Song and O. K. Chun, J. Nutr., 2011, 141, 447–452 CrossRef CAS PubMed.
- A. Vogiatzoglou, A. A. Mulligan, R. N. Luben, M. A. H. Lentjes, C. Heiss, M. Kelm, M. W. Merx, J. P. E. Spencer, H. Schroeter and G. G. C. Kuhnle, Br. J. Nutr., 2014, 111, 1463–1473 CrossRef CAS PubMed.
- S. Soares, R. M. Gonçalves, I. Fernandes, N. Mateus and V. de Freitas, J. Agric. Food Chem., 2009, 57, 4352–4358 CrossRef CAS PubMed.
- A. Rinaldi, A. Gambuti and L. Moio, Food Chem., 2012, 135, 2498–2504 CrossRef CAS PubMed.
- I. Lesschaeve and A. C. Noble, Am. J. Clin. Nutr., 2005, 81, 330S–335S CAS.
- S. Soares, R. Vitorino, H. Osório, A. Fernandes, A. Venâncio, N. Mateus, F. Amado and V. de Freitas, J. Agric. Food Chem., 2011, 59, 5535–5547 CrossRef CAS PubMed.
- V. de Freitas and N. Mateus, J. Agric. Food Chem., 2001, 49, 940–945 CrossRef CAS PubMed.
- P. Sarni-Manchado, V. Cheynier and M. Moutounet, J. Agric. Food Chem., 1999, 47, 42–47 CrossRef CAS PubMed.
- L. Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, S. Gebhardt and R. L. Prior, J. Nutr., 2004, 134, 613–617 CAS.
- S. Soares, A. Sousa, N. Mateus and V. de Freitas, Chem. Senses, 2011, 37, 191–198 CrossRef PubMed.
- A. Faria, C. Calhau, V. de Freitas and N. Mateus, J. Agric. Food Chem., 2006, 54, 2392–2397 CrossRef CAS PubMed.
- R. Goncalves, S. Soares, N. Mateus and V. de Freitas, J. Agric. Food Chem., 2007, 55, 7596–7601 CrossRef CAS PubMed.
- S. Déprez, C. Brezillon, S. Rabot, C. Philippe, I. Mila, C. Lapierre and A. Scalbert, J. Nutr., 2000, 130, 2733–2738 Search PubMed.
- V. A. P. De Freitas, Y. Glories, G. Bourgeois and C. Vitry, Phytochemistry, 1998, 49, 1435–1441 CrossRef CAS.
- S. González-Manzano, N. Mateus, V. de Freitas and C. Santos-Buelga, Eur. Food Res. Technol., 2008, 227, 83–92 CrossRef.
- I. Messana, T. Cabras, R. Inzitari, A. Lupi, C. Zuppi, C. Olmi, M. B. Fadda, M. Cordaro, B. Giardina and M. Castagnola, J. Proteome Res., 2004, 3, 792–800 CrossRef CAS.
- E. M. Coates, G. Popa, C. I. R. Gill, M. J. McCann, G. J. McDougall, D. Stewart and I. Rowland, J. Carcinog., 2007, 6 CrossRef CAS PubMed.
- C. Payne, P. K. Bowyer, M. Herderich and S. E. P. Bastian, Food Chem., 2009, 115, 551–557 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |