Thermochemical conversion of low-lipid microalgae for the production of liquid fuels: challenges and opportunities
Yu Chen
a,
Yulong Wu
*ad,
Derun Hua
a,
Chun Li
 b,
Michael P. Harold
*c,
Jianlong Wang
a and
Mingde Yang
a
b,
Michael P. Harold
*c,
Jianlong Wang
a and
Mingde Yang
a
aInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China. E-mail: wylong@tsinghua.edu.cn; Fax: +86 1062784831; Tel: +86 108979163
bSchool of Life Science, Beijing Institute of Technology, Beijing 100081, China
cDepartment of Chemical & Biomolecular Engineering, University of Houston, 4800 Calhoun, S222 Engineering Building 1, Houston, TX 77204-4004, USA. E-mail: mharold@uh.edu; Fax: +1 7137434323; Tel: +1 7137434322
dBeijing Engineering Research Center for Biofuels, Beijing 100084, China
First published on 2nd February 2015
Abstract
The development of renewable biomass energy sources has attracted attention because of the potential for a sustainable fuel with a low carbon intensity. Microalgae are considered as a third generation biofuel, and have a notable advantage over other biomass in that they do not compete with food or cropland resources. The conversion of algal biomass into liquid fuels provides a long-term sustainable option for fuels production, which can be achieved in an environmentally compatible manner. Among the microalgal conversion methods, thermochemical conversion, which can make full use of all components in the algae, is viewed as one of the best conversion methods, especially for low-lipid microalgae. This article reviews recent developments in the field of algal biomass conversion into liquid fuels, with particular attention focused on the thermochemical conversion of low-lipid microalgae. We start with a brief introduction of microalgae and its biochemical components. After an overview of the main strategies involved in algal biomass conversion, we focus on the thermochemical conversion of algae, including pyrolysis and hydrothermal liquefaction and compare the two methods in detail. In addition, the catalytic upgrading of algae-derived crude bio-oil was also examined. An assessment is made of the challenges and opportunities of a commercial-scale microalgae-to-fuels process in light of mitigating technical, environmental, and logistical issues.
1. Introduction
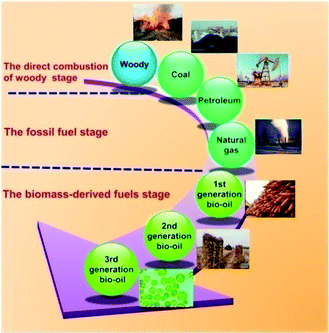
Concerns over the geopolitical and environmental implications of petroleum availability, supply, and consumption have been growing in recent years.1 With the eventual depletion of fossil fuels as a source for fuels and chemicals, the need for the development of sustainable renewable energy has become a global theme aimed at addressing the issues on climate change, energy security, and the ever-increasing demand for limited petroleum resources. This interest has prompted researchers to develop potentially viable approaches for the production of biofuel from biomass, notwithstanding recent advances in the recovery of petroleum and gas by advanced production methods like fracturing and horizontal drilling.2,3 The utilization of bioresources for the production of alternative fuels provides one of long-term sustainable options for fuels production which may be accomplished in an environmentally compatible manner for many regions of the globe.4,5 Among the renewable biomass resources, microalgae are viewed as next generation fuel feedstock due to its superior photosynthetic efficiency, higher growth rate, area-specific yield, and higher carbon dioxide utilization capabilities compared to terrestrial plants.Prior to the exploitation of low-cost fossil fuels, humankind was dependent on the direct combustion of woody biomass to meet its energy demands (Fig. 1). The fossil fuel era commenced with the use of coal for heat and electricity. The discovery of petroleum crude oil provided an inexpensive liquid fuel source that significantly improved the standard of living and, by most accounts, catalyzed the industrial revolution.6,7 Biomass-derived fuel, called “biofuel” or “bio-oil” for short, has attracting considerable attention and research activity because it is derived from renewable biomass resources and potentially relieves the entrenched global dependence on petroleum-based fuels.8–10 Moreover, biofuels are a potentially carbon-neutral feedstock. At the cornerstone of this green industrial revolution is the potential for algae as one of biofuel feedstocks.4 The concept of using microalgae to produce fuels has already been discussed for more than half a century, but a concerted effort started from the first oil crisis in the 1970s.10
Microalgae are considered as an attractive optional feedstock for several compelling reasons:11–15 (1) potential biofuel yields from certain microalgae strains are projected to be at least 60 times higher than that from soybeans, approximately 15 times more productive than terrestrial plants, and nearly 5 times of that of palm oil per acre of land on an annual basis; (2) the growth cycle of microalgae is comparatively short with biomass yield that can double within 24 h; (3) the biomass production of microalgae is 5–30 times higher than that of traditional oil crops per unit surface area; (4) microalgae can be rich in oil, over 60% by weight of dry biomass in some species; (5) microalgae does not pose a threat to traditional agricultural resources as they can be cultivated on non-arable land or waste water; (6) microalgal harvesting can be integrated with a fossil-fuel-fired power plants for capture and use of CO2 via photosynthesis. Finally, the cultivation of microalgae may be coupled with wastewater bioremediation via removing nitrogen, phosphorus, and heavy metals.
The original concept for converting microalgal biomass into biofuels involved lipid extraction for the production of biodiesel via transesterification. However, compared to low-lipid algal strains with the content of lipid less than 15 wt%, high-lipid microalgal species typically have lower biomass productivity and growth rates, and require stringent and controlled cultivation. In contrast to the transesterification process in which only the lipids in the algae are utilized, thermochemical routes involve the conversion of the entire algal cell, including the proteins, carbohydrates and lipids, into fuel oil.16 Indeed, the conversion of low-lipid microalgae into biofuel in the future is an active area of research.17–21
Microalgal proteins, lipids, and carbohydrates contain C, H, O, N and other elemental moieties. As a result, the thermochemical conversion of microalgae is a challenge for its effective utilization, C and H are the primary elemental constituents of fossil fuels and conventional refining and petrochemical processing follows from that fact. Petroleum is under-functionalized, containing mostly C and H, and therefore requires of the addition of functional groups through oxidation, amination, hydration, etc. To the contrary, microalgal biomass is over-functionalized and requires removal of functionality, particularly the O- and N-containing groups.9 Indeed, this leads to the greatest challenge on the production of liquid fuel from lignocellulosic and microalgal biomass via thermochemical conversion.22–35
There have been earlier reviews on the conversion of microalgae to biofuels.1,16,27,36–38 Several reviews on the conversion of high-lipid microalgae to biodiesels covering topics like lipid extraction and transesterification have also appeared.8,13,15 In this review, our intent is to highlight the latest developments of microalgal biomass thermochemical conversion methods and future prospects for converting low-lipid microalgae into liquid fuels. This review primarily focuses on thermochemical conversion technologies (pyrolysis and hydrothermal liquefaction) of microalgae with/without catalysts as well as the catalytic upgrading of crude bio-oils into end products. Since the overwhelming majority of transportation fuels (excess 90%) is on the basic of liquid fuels, so we focus attention on liquid fuels obtained from microalgal biomass in this review.
2. Characteristic components of microalgal biomass
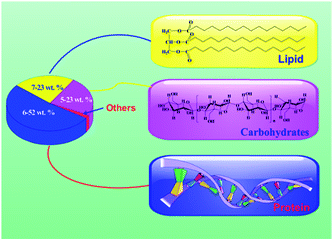
Microalgal biomass has a number of advantages over other biomass types; these advantages include higher area yields, higher oil content, lower water consumption, and ease-of-growth on non-arable lands. To this end, microalgal biomass is an intriguing feedstock for the production of biofuel.Microalgae are photosynthetic microorganisms that can produce three major biochemical components; namely, lipids, proteins and carbohydrates (Fig. 2 and Table 1). The primary elemental constituents of algae, which can be converted into fuels, are C, H, O and N. This is an important distinguishing feature of microalgae.39,40 Compared to lignocellulosic biomass, for example, these functional groups afford the potential to be made into high value-added specialty chemicals.
| Microalgae classification | Species of selected microalgae | Proteins | Carbohydrates | Lipids | Ref. |
|---|---|---|---|---|---|
| Anabaena | Anabaena cylindrica | 43–56 | 25–30 | 4–7 | 43 |
| Batrachospermum | Aphanizomenonflos-aquae | 62 | 23 | 3 | 43 |
| Chlamydomonas | Chlamydomonasrheinhardii | 48 | 17 | 21 | 43 |
| Aegagropila | Chlorella protothecoides | 53 | 11 | 15 | 44 |
| Chlorella pyrenoidosa | 71.3 | 22 | 0.1 | 42 | |
| Chlorella spp. | 30 | 15–17 | 9–13 | 45 | |
| Chlorella vulgaris | 42–58 | 12–17 | 14–22 | 21, 27, 46 and 47 | |
| Cladophora | Cladophora sp. | 25 | 25 | 6 | 48 |
| Desmodesmus | Desmodesmus sp. | 38–44 | 13–20 | 10–14 | 49 |
| Dunaliella | Dunaliellabioculata | 49 | 4 | 8 | 50 |
| Dunaliellasalina | 57 | 32 | 6 | 43 | |
| Dunaliellatertiolecta | 64 | 21 | 15 | 28 | |
| Euglena | Euglena gracilis | 39–61 | 14–18 | 14–20 | 43 |
| Chlorococcum | Chlorococcum Littorale | 38 | 23 | 16 | 51 |
| Oscillatoriopsis | Microcystisaeruginosa | 31 | 12 | 13 | 52 |
| Nannochloropsis | Nannochloropsisoculata | 42.6 | 6 | 24 | 46 |
| Nannochloropsissalina | 37 | 33 | 12 | 53 | |
| Nannochloropsis sp. | 52 | 12 | 28 | 35, 54 | |
| Nannochoropsis oc. | 57 | 8 | 32 | 21, 27 | |
| Nannocloropsisoculata | 39 | 20 | 17 | 55 | |
| Porphyridium | Porphyridiumcruentum | 28–39 | 40–57 | 9–14 | 43, 46 |
| Prymnesium | Prymnesiumparvum | 28–45 | 25–33 | 22–38 | 50 |
| Scenedesmus | Scenedesmusdimorphus | 8–18 | 21–52 | 16–40 | 50 |
| Scenedesmusobliquus | 50–56 | 10–17 | 12–14 | 43 | |
| Scenedesmusquadricauda | 47 | — | 2 | 50 | |
| Scenedesmus sp. | 60 | 10 | 20 | 56 | |
| Spirogyra | Spirogyra sp. | 6–20 | 33–64 | 11–21 | 43 |
| Spirulina | Spirulina | 55–70 | 17–23 | 4–13 | 46, 57–59 |
| Spirulina maxima | 60–71 | 13–16 | 6–7 | 43 | |
| Spirulinaplatensis | 46–63 | 8–14 | 4–9 | 43, 44, 53 and 60 | |
| S. platensis | 48 | 30 | 13 | 61 | |
| Lyngbya sp. | 30 | 13 | 1 | 48 | |
| Synechococcus | Synechoccus sp. | 63 | 15 | 11 | 50 |
| Platymonas | Tetraselmismaculata | 52 | 15 | 3 | 50 |
Lipids account for 7–23 wt% of the weight of microalgae under specific cultural conditions, such as high C/N medium, or conditions of stress.40 Microalgal lipids comprise saturated and polyunsaturated fatty acids with typically 14 to 20 carbon units for the former and >20 for the latter. Typically, lipids are in the form of triglycerides which are used to produce biodiesel via the transesterification.41 Conventional microalgae-to-biodiesel technology generally requires high-lipid strains, but these strains tend to have lower biomass productivity compared to low-lipid algal strains.42 Thus, low-lipid microalgal strains require alternative conversion strategies.
Proteins make up 6–52 wt% of the weight of microalgae.40 Proteins, which are in the form of amino acids (Fig. 2), are the main source of nitrogen in microalgae (Table 1). Purified proteins are used in the food, animal feed, health, and specialty chemical markets. On the other hand, the protein fraction means that conversion processes necessarily involve the nitrogen removal. This complicates any process of convert microalgae to liquid fuels.
Microalgae can accumulate a high carbohydrate content due to their relatively high photosynthetic efficiency.4 Carbohydrates, which are arguably the most important sources of energy and biological nutrients, comprise 5–23 wt% of the algal feedstock.40 Carbohydrates are homopolymers consisting of D-glucopyranose units linked via β-glycosidic bonds and/or α-glycosidic bonds (Fig. 2), and can be deconstructed into glucose monomers. Carbohydrates, accumulate in the plastids as reserve materials (starch), or become the main component of cell walls (cellulose, pectin, and sulfated polysaccharides).7
Overall, microalgal biomass belongs to a complex feedstock containing a large number of molecular functionalities that can be exploited in a variety of uses. Indeed, the extraction and purification of the lipid, protein, and carbohydrate components are carried out through various chemical, thermal, and microbiological processes. A major challenge is to synthesize the desired products with sufficient purity and yield. Thus, the selectivity control and feedstock utilization are paramount. As for liquid fuels production, the major challenge in microalgal biomass conversion can be described as follows: on the one hand, much of the heteroatoms (O, N and S) in the microalgal biomass are removed. On the other hand, much of C and H remain in the residue, and a large proportion of the remaining compositions are converted into liquid fuel.
For this reason, notwithstanding the obtained bio-oils from algae were similar with that of some lignocellulosic biomass via thermochemical conversion, algal bio-oils contained a number of N-compounds (most likely from protein degradation). In addition, compare to lignocellulosic biomass, there is no lignin existed in algal cell, so fewer aromatic compounds and their derivatives were present in algal bio-oils.
3. Strategies of microalgal conversion to liquid fuels
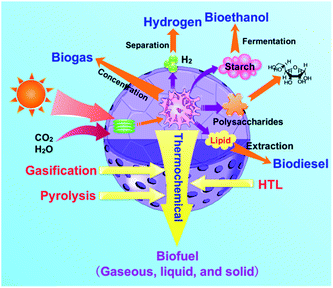
The growth of microalgae in water and its complex make-up distinguishes microalgae from conventional fossil fuels and other biomass types. With microalgal biomass being highly functionalized, conversion to a hydrocarbon-like fuel means that energy-intensive defunctionalization steps are needed. For example, the removal of oxygen requires deoxygenation through the presumed use of a reductant. Such conversion steps involve a different set of challenges compared to the refining of coal, petroleum, or natural gas. The conversion processes must be done selectively and efficiently to produce fuels with sufficient energy content while minimizing energy consumption.9 Unlike its lignocellulosic counterparts, algal biomass typically grows in water and must therefore requires extensive drying prior to further conversion.1 Moreover, its aforementioned high protein content means that conversion processes requires the removal of nitrogen.Given the unique make-up of microalgae, researchers have investigated a variety of conversion methods that concentrate on specific products.62 As depicted in Fig. 3, conversion methods include the production of biogas via concentration and/or catalysis, of biohydrogen via gasification and separation, bioethanol via fermentation, of monosaccharide via hydrolysis as platform chemicals, and of biodiesel via extraction and transesterification.8,11,13,63–78 Each approach exploits specific chemical functionality to produce different chemicals.
In contrast, thermochemical conversion has been devoted to transforming the entire algae for the production of biofuel.1,8,11,12,14,16,25–32,35,39,40,42,47–60,79,80 Thermochemical conversion can be subdivided into gasification, pyrolysis, and hydrothermal liquefaction (HTL).81,82 Pyrolysis and HTL are two key routes converting microalgae into liquid fuels. During pyrolysis, microalgae as feedstock are heated in the absence of oxygen to form bio-oil, solid char and gaseous products. On the other hand, HTL processed the microalgae at moderate temperatures produces liquid fuels in sub/supercritical water. In contrast to pyrolysis, HTL has the advantage of minimizing undesired cross-linking related reactions because the solvent dilutes the concentration of the products at relatively low temperatures.
4. Biofuel production from microalgae via thermochemical conversion
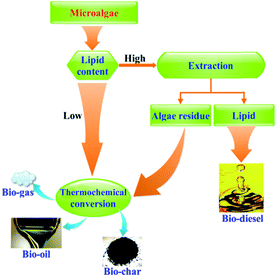
The conversion of microalgae to biodiesel via the conventional transesterification route relies on microalgae with a sufficiently high fraction of lipids.20 However, lipids comprise only a fraction of the total organic content of microalgae. The residual components consisting of proteins, carbohydrates, and unutilized lipids can be used to produce liquid, gaseous fuels and/or solid residue.83 Lipid extraction and conversion is not viable for low lipid strains. Rather, thermochemical conversion of the whole algae is carried out for biofuels production.31,36,42,83,84 Thus, thermochemical conversion is an option to process low-lipid microalgae or post-extraction residues of high-lipid microalgae (Fig. 4).The thermochemical conversion (pyrolysis and HTL) of microalgae spans thermal decomposition and chemical reformation of the organic matter into biofuel.85 A comparison of pyrolysis and HTL is provided in Table 2.38 Pyrolysis refers to the anaerobic thermal decomposition of organic compounds into a mixture of gases, liquid, and chars.86 Traditional pyrolysis is noncatalytic while recent attention has focused on the downstream catalytic upgrading of pyrolysis oils. HTL involves the reaction of microalgae in a solvent at elevated temperatures and pressures with or without catalyst.31 Pyrolysis is characterized by short gas residence times, operation at atmospheric pressure and relatively high temperatures. Prior to the pyrolysis, the algal feedstock must undergo dewatering and drying steps, which includes sedimentation, flocculation, dissolved air flotation, filtration, and centrifugation. Drying is one of most dominant costs for algae harvest and may account for 30% of the total product costs, and the power consumption was equivalent to 15.8% of the energy of the recovered hydrocarbon. Energy costs climb steeply when the concentration of the slurries increases. In contrast, HTL is usually performed at lower temperatures with longer residence time and much higher pressure. HTL converts the algal feedstock into biofuel in an aqueous phase, obviating the dewatering and drying procedures. Therefore, HTL is ideally suited to conversion of wet microalgae because it is tolerant to the high moisture content of the feedstock.25,87
| Methods | Treatment condition/requirement | Reaction mechanism/process description | Technique feasibility | |
|---|---|---|---|---|
| Superiority | Drawback | |||
| a The bio-oil yield from pyrolysis includes the aqueous fraction whereas HTL excludes the water soluble products (aqueous fraction). | ||||
| Pyrolysis | Relatively high temperature (723–773 K); short residence time (∼1 s); atmosphere pressure; drying necessary | Light small molecules are converted to oily products through homogeneous reactions in the gas phase | High biofuel yield up to 80 wt% on dry feed; low capital cost | Feedstock need to be dried prior to use. Poor biofuel quality obtained |
| HTL | Low temperature (573–673 K); long residence time (0.2–1.0 h); high pressure, (5–20 MPa); drying unnecessary | Occurs in aqueous medium which involves complex sequences of reactions | Available for commercial use; better quality of biofuel (high HHV, low moisture content) | Relatively low biofuel yield (20–60 wt%); need high pressure equipment, thus higher capital cost |
In addition, HTL results in a higher quality of biofuel whereas pyrolysis, with the exception of fast pyrolysis, results in higher yield (Table 2).38
This section focuses on pyrolysis and HTL process for the production of bio-oil from microalgae in detail. Published studies focused on microalgal conversion via pyrolysis and HTL to produce bio-oil with or without catalysts fall into this scope. Aside from the conventional thermochemical conversion, other assisted technologies, such as microwave assisted process and co-process will also be presented for comparison.
4.1 Pyrolysis of microalgae for bio-oil production
Pyrolysis is a thermochemical change of organic matter in a heated enclosure, usually in an oxygen-absent or very low oxygen level environment, to form a mixture of gases, liquid, and solids residue.86 Most earlier applications have utilized coal and peat as feedtocks while lignocellulosic biomass has attracted attention in recent years.88–96 More recent studies have shown that pyrolysis of microalgae can produce bio-oil that is superior to bio-oil produced from lignocellulosic biomass.1,11–19 The pyrolysis of microalgae aims at maximizing the production of energetically exploitable liquid and gaseous products.An optional reaction for microalgae pyrolysis is conducted in a fixed-bed reactor that is externally heated by an electrical furnace with the temperature measured by a thermocouple positioned inside the bed (Fig. 5).56 The experiment steps are described as follows: (1) the reactor was filled with a certain amount of microalgal biomass that had been dried and ground to a particle size. (2) The reactor system was purged with carrier gas (e.g., N2) which was also used during the reaction. (3) With the carrier gas flown, the reactor system was heated at a certain heating rate until reached the reaction temperature, and then held for some minutes. (4) The product vapors were collected through condensation in flasks cooled by an ice bath. (5) The aqueous and organic phases were separated, and then detected using some analysis methods (such as GC/MS, FT-IR, and elemental analysis) to evaluate the bio-oil composition.
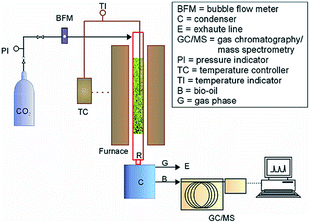 | ||
| Fig. 5 Schematic reactor system for microalgae pyrolysis.56 Copyright 2012 Elsevier. | ||
Thermogravimetric analysis (TGA) is an established method for studying the thermal degradation mechanisms and kinetics of microalgae.40,44,45,97–103 TGA provides semi-quantitative information about the pyrolysis temperature and kinetics, and phase distribution of the products, notwithstanding its rather unrealistic gas–solid contacting design. To illustrate the information gleaned from TGA, the pyrolysis of two microalgal species (Spirulina platensis and Chlorella protothecoides) were carried out by a conventional temperature ramp.44,97 TGA revealed the maximum weight loss from these two microalgae occurred over a several hundred degree temperature range (∼420–830 K) with minor differences for each species. An increase in the heating rate caused a shift in the degradation to higher temperatures which was interpreted with a simple model that inferred a decrease in the activation energies for the devolatilization stage and an increase in both the instantaneous maximum and average reaction rates.
In general there are three primaries stages that occur during the pyrolysis of microalgae. Zou et al.98 carried out the thermal pyrolysis of D. tertiolecta. They affirmed that moisture was removed in the first stage; most of the pyrolysis occurs in the second stage in which most of the organic material was decomposed based on the largest decrease in mass; remaining solid was slowly decomposes in the third stage. The initial temperature of pyrolysis and the temperature at which the pyrolysis rate reaches the peak value shifts to the higher range as the heating rate increased. Similar pyrolysis features have been reported for Nannochloropsis gaditana, Chlorella spp., Nannochloropsis, Potamogeton crispus, Sargassum thunbergii, C. vulgaris, and Nannochloropsis sp.40,45,99–101
The pyrolysis behavior of the common Chlorella species obtained using TGA show that the volatile species consist primarily of water and CO2, as well as H2 at high temperatures.102 The thermal pyrolysis of Nannochloropsis sp. indicated the different biochemical components of the microalgae in the absence of a catalyst seem to be decomposed in the following order: carbohydrates, proteins, and lipids.99 The addition of catalysts with the microalgae can have an important effect in the presence of Na2CO3 during the pyrolysis process, the main weight decrease shifted to a lower temperature. This indicated that Na2CO3 may affect the decomposition of carbohydrates and proteins but not that of lipids. The Na+ from Na2CO3 can penetrate the biomass and break the hydrogen bonds, enabling pyrolysis to occur at lower temperatures.
The thermal behavior of six microalgal species (Tetraselmischui, Chlorella like, C. vulgaris, Chaetocerous muelleri, D. tertiolecta and Synechococcus) showed the results as follows: First, the ratio of evolved liquid, gas and char products varied markedly across all species with temperatures up to 773 K; second, the rate and temperature of evolution of these fractions was also inconsistent; third, the mix of combustible volatile gas compounds that evolved with temperature was also variable, resulting in differences in the its energy content (higher heating value, HHV).103
In summary, the pyrolysis behavior of microalgae obtained from TGA can provide useful information for laboratory-scale pyrolysis. However, the measurements on pyrolysis behavior of microalgae have its limitations due to the unrealistic gas–solid contacting. These limitations activate the incentive to devise and apply alternative analysis methods that enable bridging of the gap between the lab- and full-scale pyrolysis, thereby enabling a fundamental study of pyrolysis.
| Process types | Reaction condition | ||||
|---|---|---|---|---|---|
| Residence time | Heating rate/K s−1 | Temperature/K | Main products | ||
| Slow pyrolysis | Carbonization | Hours–days | Very low | 573–673 | Charcoal |
| Conventional pyrolysis | 5–30 min | 0.1–1 | 573–973 | Gas, liquid and charcoal | |
| Fast pyrolysis | Fast pyrolysis | 0.5–5 s | 1–200 | 773–1073 | Liquid |
| Flash pyrolysis | <0.5 s | >103 | 823–1273 | Liquid and/or gas | |
(1) Slow pyrolysis. In addition to the relatively slow heating rate, slow pyrolysis is characterized by longer gas residence time.117 Slow pyrolysis of microalgae results primarily in the production of biochar and pyrolysis gas.102,105 CH4 and CO2 are the major components in the gaseous product.86 Some examples follow to illustrate.
Slow pyrolysis of C. protothecoides resulted in a bio-oil yields exceeding 40 wt%.106 The gaseous product yield generally increased with temperatures and residence time due to the secondary reactions. When Spirulina sp. was used as feedstock via slow pyrolysis, the optimized temperatures with the highest maximum biochar and bio-oil yields were 773 and 823 K, respectively.107 In addition, the bio-oils obtained from slow pyrolysis of Nannochloropsis sp. Residue mainly consisted of long-carbon chain compounds with various terminal groups with an oxygen content of 30.1 wt% and a HHV of 24.6 MJ kg−1.108
Harold et al. compared the slow pyrolysis of Scenedesmus sp. with that of duckweed.56 Scenedesmus sp. afforded a higher bio-oil yield than duckweed, whereas microalgal bio-oil had a higher HHV of 19 MJ kg−1 than that of duckweed bio-oil (15 MJ kg−1). The authors stated that the thermolysis of proteins, carbohydrates and lipids required less stringent conditions than that of lignocelluloses and resulted in a product with higher HHV. In addition, they affirmed several reactions during the thermolysis process including deamination, direct methylation, decarboxylation (DCO), dehydration, decarbonylation, cyclization, dimerization, and homonolysis. Another important reaction occurred in this process is Maillard reaction, which converts carbohydrates and proteins to form amadori compounds, as depicted in Fig. 6.56
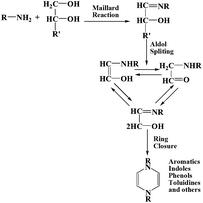 | ||
| Fig. 6 Proposed reaction mechanism leading to the formation of amadori compounds.56 Copyright 2012 Elsevier. | ||
Slow pyrolysis has the noted disadvantage of leading to secondary cracking, condensation and polymerization of pyrolysis products. These reactions lead to decreasing the bio-oil yield and have adverse effects on the bio-oil properties. Moreover, the lower HHV and the longer residence time tend to increase the energy requirements.
(2) Fast pyrolysis. Since the aim for microalgal pyrolysis is the production of organic liquid phase (biofuel), fast pyrolysis is recommended (Table 3). The achievement of heating rates as high as 200 K s−1 requires high operating temperatures, short residence time, and fine particles (<1 mm).104
The fast pyrolysis of dried, ground Scenedesmus sp. resulted in a bio-oil which rivaled that from lignocellulosic feedstocks.109 Moreover, the bio-oil yield increased with temperature up to a point and then decreased.110 The rather high bio-oil yield from microalgae suggests that fast pyrolysis is a potential method for converting algae to liquid.105 When the conversion of C. prothothecoides and M. aeruginosa was carried out with fast pyrolysis, the bio-oil yields were 18 and 24 wt%, respectively.52 Additionally, the bio-oil from fast pyrolysis of microalgae has a HHV of 29 MJ kg−1, which is about 1.4 times of that of wood. Liquid fuels from fast pyrolysis of microalgae may be used in many applications as direct substitutes for conventional fuels.52
The bio-oil yield ranged from 25–30 wt% with a HHV of 25 MJ kg−1 in the fast pyrolysis of microalgae in a falling solids reactor.111 Fig. 7 shows the GC-MS-classified organic fractions of bio-oils obtained during pyrolysis in three atmospheres (N2, steam and CO2). The data indicate that the pyrolysis atmosphere has an important effect on the product distribution, and the presence of steam increases the fraction of hydrocarbons while decreases the oxygenate fraction. The C fraction from the steam swept pyrolysis exceeds that from the N2-swept pyrolysis while the O fraction is lower. These trends reflect in a higher HHV for the bio-oil during the steam swept process. The authors speculated that the reactions occurring during steam pyrolysis are presumably steam reforming and deoxygenation.111
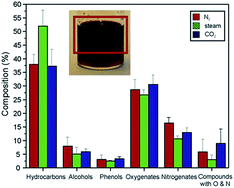 | ||
| Fig. 7 Bio-oil composition of pyrolysis employed at three atmospheres: N2, steam, and CO2 (ref. 111). Copyright 2012 Elsevier. Process conditions: 773 K, N2 flow = 250 mL min−1, algae mass = 7 g, dp < 90 mm. | ||
The pyrolysis temperature plays a crucial role on the pyrolysis product distribution of blue-green algae blooms.112 The maximum bio-oil yield of 55.0 wt% was obtained at a pyrolysis temperature of 773 K, particle size below 0.25 mm and a sweep gas flow rate of 100 mL min−1. The bio-oil has a HHV of 31.9 MJ kg−1 and an O/C molar ratio of 0.16 under optimum conditions. These results showed that the pyrolysis of algal biomass is a promising process for producing renewable fuel while improving the quality of a freshwater lake.112
Pyrolysis through microwave-assisted power has been proposed for both microalgae and their extraction residues to obtain bio-oil.113 Microwave-assisted pyrolysis (MAP), initially developed by Tech-En Ltd. in Hainault, UK, has been investigated in recent decades.114,115 Compared to the traditional processes, MAP offers several advantages including uniform internal heating of large biomass particles, ease of control, no need for agitation or fluidization and fewer particles (ashes) in the bio-oil.116
Noteworthy is the enhancing effect of the microwave power on the pyrolysis product yield. A maximum bio-oil yield of 32.0 wt% was obtained at a microwave power of 750 W in the MAP of Chlorella sp. When the material was changed into C. vulgaris, it was found that a microwave power of 2250 W gave the highest bio-oil yield of 74.9 wt%; a bio-oil yield as high as 87.4 wt% was obtained when activated carbon was added as a catalyst.117 Notwithstanding current MAP techniques offering numerous advantages and showing excellent potential for enhancing microalgal bio-oil yield, the growth of industrial microwave heating applications is hampered by the lack of knowledge on the microwave systems and commercial necessary equipment for these pyrolysis processes. In addition, the economic assessment of the MAP has yet not been conducted.
Fast pyrolysis requires a reactor configuration in which the residence time of microalgae is of order only a few seconds. As mentioned earlier, fast pyrolysis seems to be a viable technique for future replacement of fossil-fuel with biomass-derived liquid fuels because of the potential for high biomass-to-liquid yield. However, some technical challenges need to be solved because bio-oils from direct pyrolysis are acidic, unstable, viscous, and contain solids and chemically dissolved in water. Therefore, the bio-oil must be upgraded via hydrogenation or catalytic cracking to decrease oxygen content and remove alkalis.
Compared to the noncatalytic pyrolysis of algal biomass, catalytic pyrolysis can not only upgrade the quality of bio-oil but also adjust the components of bio-oils to meet different demands.108 This may be accomplished either by mixing biomass with the catalysts118 or flowing pyrolysis vapors over a catalyst positioned downstream from the pyrolysis zone. Catalytic pyrolysis generally produces biofuel of enhanced quality (higher HHV, lower oxygen content, and higher aromatic hydrocarbon content) even at atmospheric pressure without the need for a reductant, which makes this a cost-effective upgrade.119 Previously, most of the applied catalysts focused on molecular sieves in the catalytic pyrolysis process of algae. However, the investigation of other catalysts may be considered to ameliorate biofuel quality.120 Here we highlight some recent examples.
(1) Alkalis salt catalysis. Babich et al. studied the pyrolysis of Chlorella both with and without Na2CO3 as the catalyst.102 The presence of the Na2CO3 catalyst lowered the initial degradation temperature, and produced a bio-oil with lower acidity and higher HHV than bio-oils produced without the catalyst. However, the Na2CO3 also promoted the gas yield and reduced the liquid yield.
(2) Molecular sieve catalysis. Early in 1990, Milne et al. first proposed the catalytic conversion of entire microalgae over HZSM-5molecular sieve, but the obtained results were ambiguous.121 The formation of high value-added aromatics is enhanced considerably, which can be attributed to the acidity of the zeolite catalysts in the catalytic pyrolysis of Laminaria japonica over microporous zeolite catalysts (HZSM-5, Hβ and HY).122 HZSM-5, which, together with its Brönsted acidity and specific pore structure, showed the highest selectivity for aromatic production. Compared to noncatalytic pyrolysis, the catalytic pyrolysis of microalgae produced lower bio-oil yields because of the catalytic cracking of bio-oil compounds to form gaseous products.122 Similar results were reported by Wang,47 Du123 and Gopakumar124 when HZSM-5 was used for the catalytic pyrolysis of C. vulgaris. An increase of the aromatic hydrocarbon yield of 0.9 to 25.8 wt% was achieved while the oxygen content in turn decreased from 30.1 to 19.5 wt% and the HHV increased from 24.4 to 32.2 MJ kg−1.124 The catalytic pyrolysis of microalgal biomass produces more monocyclic aromatics than that of lignocellulosic biomass.47 Pan et al. pyrolyzed Nannochloropsis sp. using variable amounts of HZSM-5 over a range of temperatures.108 The catalyst increased the HHV of the bio-oil from 24.6 to 32.7 MJ kg−1. The bio-oil obtained from catalytic pyrolysis is rich in aromatic hydrocarbons based on the GC-MS results.
A detailed study comparing noncatalytic and catalytic pyrolysis using exchanged cations ZSM-5 catalysts was carried out by Campanella and Harold.111 Noncatalytic pyrolysis gave the highest total liquid content, whereas catalytic pyrolysis resulted in the highest hydrocarbon fraction. A comparison of four exchanged ZSM-5 catalysts (H-, Fe-, Cu- and Ni-) showed differences in the bio-oil yield and composition (Fig. 8).111 Among the studied catalysts, H-ZSM-5 gave the largest enhancement in the liquid product yield.111 Fig. 8a shows the effect of these catalysts on the bio-oil yields under identical operating conditions. The solid residue yield remained unchanged or slightly decreased. The volatile product yield increased at the expense of a decrease in the pyrolysis oil (bio-oil plus aqueous fraction). These results provide evidence for desired deoxygenation chemistry that produces CO, CO2 and light hydrocarbons. Further, GC-MS measurements demonstrated that the zeolites catalyst enhanced the energy content associated with the more favorable bio-oil composition (Fig. 8b). Notable trends included a decrease in the fraction of oxygenated species and an increase in the fraction of hydrocarbons; meanwhile, the yield of nitrogen compounds remained the same or slightly decreased. Compared to noncatalytic pyrolysis, the catalytic pyrolysis provided an increase in phenols, which are high value-added chemicals, and could increase the attractiveness of the catalytic pyrolysis of microalgae.111 In addition, the enhanced aromatic fraction yield may owe to Dielse–Alder and condensation reactions.
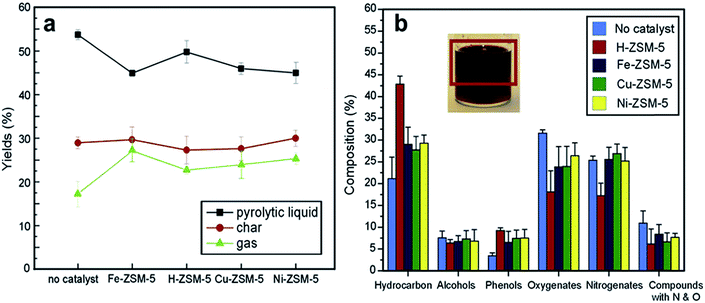 | ||
Fig. 8 Experimental results of catalytic pyrolysis reactions conducted using different zeolites.111 Copyright 2012 Elsevier. (a) Effect of catalyst on product yield in catalytic pyrolysis reactions. (b) Bio-oils product distribution. Process conditions: 773 K, N2 flow = 250 mL min−1, algae mass = 7 g, WHSV = 13.5 h−1, GHSV = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, residence time ∼1.5 s. 000 h−1, residence time ∼1.5 s. | ||
In summary, the heteroatom derived from the microalgae biochemical composition can only be partly removed in catalytic pyrolysis, while the noncatalytic pyrolysis followed by bio-oil upgrading (two-stage method) can remove almost all the heteroatoms to satisfy the requirements for use as transportation fuel. However, the two-stage method presents some disadvantages, including numerous treatment steps, high storage cost, and low bio-oil yield, etc. Therefore, both of the two methods have their advantages and disadvantages.
Tang et al. studied the co-pyrolysis characteristic of microalgae and municipal solid waste under N2/O2 and CO2/O2 atmospheres using TGA.132 As the blending ratio of microalgae increased from 10 to 70 wt% under a N2/O2 atmosphere, the volatile release temperature decreased from 542 to 520 K, the temperature at the maximal peak decreased from 583 to 561 K, the maximum rate of weight loss decreased from 11.94 to 7.88 wt% and the residual weight decreased from 30 to 20 wt%.
The results from a TG- and modeling-based study using fresh water algae Chlorococcum humicola and a Victorian brown coal and their blends at different proportions showed that a smaller amount of algae can be mixed with coal without significantly changing in the pyrolysis characteristics.133 Coal was mixed with algae to produce a slurry and then the combustion behavior of the coal-water slurry was investigated by Li et al.134 Yuan et al. studied the rapid pyrolysis of the aquatic biomass (blue-green algae and water hyacinth) with two coals (bituminous and anthracite).135 During the co-pyrolysis of algal biomass and coal, the interactions between algae and coal decreased char-N yields and increased volatile-N yields, but the total yields of NH3 + HCN decreased. HCN formations consistently decreased, whereas NH3 formations only decreased in the high-temperature range but increased in the low-temperature range.
• Energy content. While compared to other biomass sources, the energy content of algae on a dry basis is nominally half that of a fossil fuel. The rather high oxygen content is primarily responsible. The removal of oxygen through chemistries like decarbonylation is essential to reduce the reductant requirements for deoxygenation.
• Water content. Algae grow in water and therefore require dewatering and drying prior to pyrolysis. The latter is particularly energy intensive. The use of a fraction of the water during the pyrolysis as a source of hydrogen through reforming of pyrolysis products is one approach.
• Use of photo bioreactors (PBR). One way to address the land requirement is to grow algae in transparent vertical columns. Designs would be needed to maximize the utilization of sunlight and minimize the materials cost. The use of polymeric materials that withstand photocatalytic degradation is essential. The materials cost of the PBR is a non-negligible factor in the overall economics.
• Logistical issues. Notwithstanding the land and water requirements for growth, the infrastructure needed for harvesting and conversion would require a massive effort and investment. Bio-oil production facilities should be located in close proximity to the lands producing the algal biomass. A stabilized bio-oil could then be transported to refineries for conversion to transportation liquids. This poses a trade-off between transportation costs and economies-of-scale afforded by larger pyrolysis facilities.
4.2 HTL of microalgae for bio-oil production
As described above, microalgal pyrolysis converts whole algae into liquid fuels.20 However, a large amount of water accompanies algae with typical algal cell density of 1 g L−1.1 As a result, the economics of microalgal pyrolysis are undermined by the costly dewatering and drying steps.28As alternative to pyrolysis is hydrothermal liquefaction (HTL) which involves conversion of microalgae in slurry comprising a liquid solvent and is carried out at moderate temperatures and sufficient pressure to keep the solvent in the liquid phase. HTL is of interest because it eliminates the need to expend the energy to dewater and dry algae, as required in other thermochemical conversions.140 To this end, the conversion of algae via HTL has received increasing interest in recent years even though biomass HTL is a rather mature technology, first described in the 1940s, with technology improvements in the 1980s by Shell researchers.141
HTL utilizes a variety of solvents, including water as the reaction medium. The use of water of course presents several advantages over other solvents because it is ecologically safer, cheaper, and readily available, not to mention that it is the growth medium for algae.142 It is noted that the ionic product of water under high temperature and pressure conditions below the critical point of water is up to three orders of magnitude higher than that under ambient conditions (Fig. 9).143 A high ionic product is favorable for acid or base catalyzed reactions. In addition, water can act as an acid or base catalyst precursor due to the relatively high concentrations of H3O+ and OH− ions from the self dissociation.143 When water is heated and compressed, the hydrogen bonds are weakened, resulting in a change in dielectric constant, acidity, and polarity, and increasing the reactivity of water. For example, the dielectric constant of water decreases from 78.85 to 13.96 when the temperature increases from 25 to 250 °C, resulting in the transition of water molecules from very polar to fairly nonpolar.144 The dissociation constant of water (Kw) increases from 10−14 to 10−11 just below 250 °C, resulting in an enhancement in the rates of acid- and base-catalyzed reactions in water.145
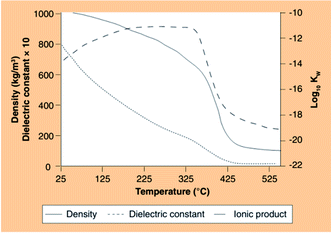 | ||
| Fig. 9 Density, static dielectric constant at 30 MPa and ionic product of water at 25 MPa.143 Copyright 2012 Future Science Group. | ||
HTL of microalgae proceeds through a multi-step procedure as depicted in Fig. 10, and are as follows. (1) During the initial stages the algal cell membrane and wall are disrupted chemically in the high temperature and pressure water. Numerous reactions between organic compounds in the cell membrane and wall occur, and some small molecules are produced. (2) Once the microalgae are lysed, the intracell components, lipids, proteins, and carbohydrates, participate into the reaction process. Hydrolysis and depolymerization occur, converting carbohydrates into monosaccharides and polysaccharides, proteins into peptides and amino acids, and lipids into fatty acids and glycerol. (3) Repolymerization/self-condensation reactions occur. This includes the conversion of lipids into fatty acids, proteins into nitrogen heterocycles, pyrroles, and indoles; and carbohydrates into cyclic ketones and phenols. Others chemical reactions such as Maillard reaction between the smaller molecules may be also present at this stage. (4) Following the liquefaction process, algae are eventually converted into a series of products including liquid, gas and solid residue. The long-chain nonpolar molecules generally named as bio-oil are formed. Short chain polar molecules are dissolved into water medium and the formed aqueous phase, which is mostly used to cultivate microalgae. The gaseous products, mainly CO2, are directly vented to the atmosphere in most cases.
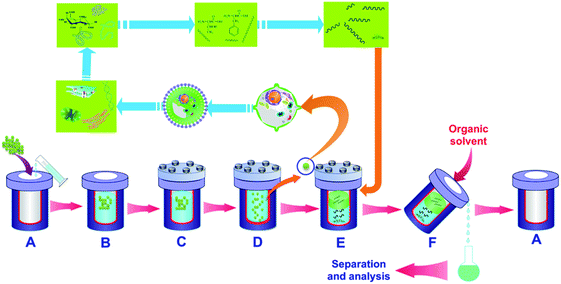 | ||
| Fig. 10 Experimental procedure of HTL for microalgae in closed batch reactor (A) reactor, (B) charging, (C) sealing, (D) heating, (E) cooling, (F) washing. | ||
In real HTL process, bio-oil can be separated easily from water phase with the addition of the organic solvents (such as trichloromethane, dichloromethane, tetrahydrofuran, and n-hexane). The separation procedure of HTL products is described in published papers.42,49,53,54
Microalgal derived HTL bio-oil has several desirable attributes. First, the HTL conversion of microalgae into bio-oil has an efficiency of 30–75% and a net positive energy yield that is 3–10 times of the input heat energy.146 Based on the principle of green chemistry, Zhang et al. proposed the concept of Environment-Enhancing-Energy (E2-Energy) to integrate bio-oil production and wastewater treatment (Fig. 11).42 According to E2-Energy, microalgae are grown to serve as a wastewater treatment method to uptake nutrients and capture CO2 from HTL products.12 Subsequently, the resulting algae are further converted into bio-oil via HTL. The algae species that grow in the post-HTL water are expected to have low lipid content because the N and C contents of the HTL aqueous stream are high.42 That said, the bio-oil obtained from the HTL of microalgae still contains a fraction of O and N. Such bio-oil has a lower quality and HHV compared to the actual transportation fuel.
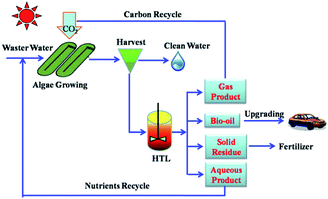 | ||
| Fig. 11 Concept of Environment-Enhancing-Energy (E2-Energy) technology.42 Copyright 2011 Royal Society of Chemistry. | ||
To improve the quality of bio-oil, two methods have been developed. One method involves HTL with homogeneous and/or heterogeneous catalysts. The other method involves the use of organic solvents or co-processing with other substances for microalgal HTL. In this section, we highlight the developments of noncatalytic HTL firstly, followed by catalytic HTL, and then by HTL in co-solvents. In addition, selected contents that elucidate the mechanism of microalgal HTL also fall within scope of this section.
| Type | Ref. | Catalysts | Feedstocks | Conditions | Max. bio-oil yield (wt%) | Max. HHV, (MJ kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| Reaction medium | Temperature (K) | Holding time, (min) | ||||||
| a Microwave-assisted liquefaction. | ||||||||
| Noncatalytic liquefaction (direct liquefaction) | 148 | — | Dunaliella tertiolecta | Water | 633 | 30 | 36.9 | 26.6 |
| 54 | — | Nannochloropsis sp | Water | 473–773 | 60 | 43.0 | 39.0 | |
| 49 | — | Desmodesmus sp. | Water | 448–723 | 5–60 | 49.0 | 36.0 | |
| 53 | — | Spirulina | Water | 493–648 | 30 | 38.0 | 35.2 | |
| Nannochloropsis salina | 46.0 | 38.1 | ||||||
| 149 | — | Nannochloropsis sp. | Water | 873 | 1 | 66.0 | 37.0 | |
| 86 | — | Scenedesmus | Water | 573 | 30 | 45.0 | 35.5 | |
| Spirulina | 31.0 | 35.8 | ||||||
| 42 | — | Chlorella pyrenoidosa | Water | 553 | 120 | 39.4 | 35.4 | |
| 150 | — | Chlorella pyrenoidosa | Water | 553 | 120 | 39.4 | 35.4 | |
| 58 | — | Spirulina | Water | 573 | 30 | 32.6 | 34.7 | |
| 151 | — | Spirulina platensis | Water | 623 | 60 | 39.9 | 39.9 | |
| 61 | — | Spirulina platensis | Water | 623 | 60 | 41.0 | 34.2 | |
| 57 | — | Spirulina | Ethanol | 633 | — | 35.4–45.3 | 32.6 | |
| 59 | — | Spirulina | Ethanol | 653 | 20 | 54.0 | 38.3 | |
| Methanol | 55.1 | 39.8 | ||||||
| 1,4-Dioxane | 56.6 | 36.8 | ||||||
| 152 | — | Chlorella pyrenoidosa | Ethanol | 443–643 | 5–120 | 64.6 | 38.9 | |
| Homogenous catalysis | 29 | Na2CO3 | Botryococcus braunii | Water | 473–613 | 60 | 64.0 | — |
| 28 | Na2CO3 | Dunaliella tertiolecta | Water | 473–613 | 5 and 60 | 43.8 | 36.0 | |
| 153 | Na2CO3 | Enteromorpha prolifera | Water | 493–593 | 5–60 | 23.0 | 30.0 | |
| 33 | Na2CO3 | Microcystis viridis | Water | 573 and 613 | 30 and 60 | 33.0 | 30.0 | |
| 32 | Na2CO3 | Dunaliella tertiolecta | Water | 633 | 50 | 25.8 | 30.7 | |
| 31 | Na2CO3 | Chlorella vulgaris | Water | 573 and 623 | 60 | 27.3 | 37.9 | |
| KOH | ||||||||
| CH3COOH | Spirulina | 20.0 | 39.9 | |||||
| HCOOH | ||||||||
| 27 | Na2CO3 | Chlorella vulgaris | Water | 623 | 60 | 35.8 | 37.1 | |
| HCOOH | Nannochloropsis occulata | 34.3 | 34.3 | |||||
| Porphyridium cruentum | 20.0 | 36.3 | ||||||
| Spirulina | 29.0 | 36.8 | ||||||
| 60 | Na2CO3 | Spirulina platensis | Water | 573–623 | 30–60 | 51.6 | 36.3 | |
| Ca3(PO4)2 | 34.5 | 38.4 | ||||||
| NiO | 30.2 | 35.1 | ||||||
| 57 | FeSO4 | Spirulina | Ethanol | 633 | — | 46.0 | 37.1 | |
| FeS | ||||||||
| Na2CO3 | ||||||||
| NaOH | ||||||||
| 27 | HCOOH | Chlorella vulgaris | Water | 623 | 60 | 27.0 | 33.2 | |
| Spirulina | 29.0 | 35.1 | ||||||
| Nannochloropsis occulta | 26.0 | 39.6 | ||||||
| Porphyridium creuntum | 27.1 | 36.3 | ||||||
| 154 | H2SO4 | Sargassum polycystum | Ethylene glycol | 443 | 15 | 87.7a | — | |
| 155 | H2SO4 | Dunaliella tertiolecta | Ethylene glycol | 443 | 33 | 45.0 | 28.4 | |
| Heterogeneous catalysis | 86 | HZSM-5 | Chlorella pyrenoidosa | Ethanol | 473–573 | 30 | 71.3 | 36.2 |
| Raney-Ni | ||||||||
| 35 | Pd/C | Nannochloropsis sp. | Water | 623 | 60 | 57.0 | 38.0 | |
| Pt/C | ||||||||
| Ru/C | ||||||||
| Ni/SiO2–Al2O3 | ||||||||
| CoMo/γ-Al2O3 | ||||||||
| Zeolite | ||||||||
| 156 | Ni/REHY | Dunaliella salina | Water | 473 | 60 | 72.0 | 30.1 | |
| 21 | Co/Mo/Al2O3 | Chlorella vulgaris Nannochloropsis occulta | Water | 623 | 60 | 38.7 | 39.7 | |
| Ni/Al/Al2O3 | 30.0 | 42.0 | ||||||
| Pt/Al/Al2O3 | 38.9 | 38.2 | ||||||
| 157 | Fe(CO)5–S | Spirulina | Water | 573 and 613 | 30 and 60 | 78.3 | 33.0 | |
The produced gas from HTL consists mainly of methane and carbon dioxide, such as the study of HTL of B. braunii.163 Similar results were reported by Minowa et al.28 who obtained a bio-oil yield of 37 wt% with a HHV of 36 MJ kg−1 from D. tertiolecta. Other low-lipid microalgae, like Spirulina, were used as the feedstock, and the biofuel yield could reach as high as 78.3 wt%.157 A bio-oil yield of 49 wt% was obtained from the HTL of Desmodesmus sp. as the feedstock. About 75% of the HHV (22–36 MJ kg−1) in microalgae was transformed into bio-oil.49
Researchers have reported that the highest bio-oil yields are obtained during HTL under subcritical conditions. Brown et al. converted the microalga Nannochloropsis sp. into bio-oil via HTL at different temperatures.54 The highest bio-oil yield of 43 wt% was obtained at 623 K which corresponded to subcritical water. The bio-oil yields decreased from this maximum value in the 673–723 K temperature range. By 773 K, the bio-oil yield was nearly half the maximum value as a result of oil-range molecules reacting to form lighter and more volatile compounds which are not captured in the oil fraction. Moreover, at higher temperatures (supercritical conditions), higher molecular-weight compounds derived from oil-range molecules react together to form solid products.54
Zou et al. studied in detail the factors influencing HTL of the microalga D. tertiolecta for the production of bio-oil under various conditions.148 The maximum bio-oil yield was approximately 36.9 wt% at a reaction temperature of 633 K and a holdup time of 30 min, with a feedstock ratio of materials to water of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The empirical formula of bio-oil with a HHV of 26.6 MJ kg−1 was established as CH1.38O0.43N0.07, and the bio-oil included species such as hexadecanoic acid, palmitamide, and fatty acid methyl esters.148
10. The empirical formula of bio-oil with a HHV of 26.6 MJ kg−1 was established as CH1.38O0.43N0.07, and the bio-oil included species such as hexadecanoic acid, palmitamide, and fatty acid methyl esters.148
The HTL of low-lipid microalga C. pyrenoidosa resulted in a peak bio-oil yield of 65.4 wt% when carried out at 553 K and a reaction time of 120 min. The resulting HHV was 35.4 MJ kg−1, which increased to 38.5 MJ kg−1 at 573 K and 30 min reaction time, suggesting lower O and N contents in the bio-oil.42
In summary, studies of HTL of microalgae shows that a rather high bio-oil yield (20–66 wt%) can be obtained, which is a highly viscous bio-oil with are relatively high N content of 1–5 wt% and a HHV of 20–40 MJ kg−1. The optimum operating conditions for obtaining the maximum bio-oil yield is in the 573–623 K temperature range with reaction times of 15–120 min. However, the operating conditions are highly specific to strain and system. To obtain a bio-oil with lower nitrogen content, lower reaction temperature and shorter reaction time should be used or the protein fraction should be removed prior to HTL. Most of the aforementioned studies have used small bench-scale reactors with capacity less than 10 g of microalgae as feedstock. Jena et al. conducted a relatively large scale experiment with S. platensis in a 1.8-L batch reactor.151 A reaction temperature of 623 K, holdup time of 60 min, and solids content of 20 wt% were identified as the optimal conditions for achieving the bio-oil yield of ∼40 wt%. Meanwhile, 98.3 wt% of carbon was converted, and the obtained bio-oil had a HHV of 39.9 MJ kg−1.
HTL is typically performed with slow heating and/or long reaction time (tens of minutes or longer). However, some recent results have suggested that shorter reaction times may be sufficient. A decrease in the reaction time would greatly reduce the reactor volume required for continuous bio-oil production, subsequently reducing the capital costs of such a process. Savage et al. investigated the fast HTL of the green marine alga Nannochloropsis sp. at batch reaction times of 1, 3, and 5 min within the temperature range of 573–873 K. Conventional liquefaction was carried out for 60 min at the same temperature range as a comparison.149 The bio-oil yield of 66 wt% was obtained at a reaction time of 1 min and temperature of 873 K. This yield exceeded any previous report on the HTL of the same algal species. The bio-oil produced by fast HTL has carbon contents and HHV similar to those produced by the traditional isothermal liquefaction process, which involves treatment for tens of minutes. Moreover, the authors affirmed that the reaction ordinate is a useful parameter for interpreting the results from algae liquefaction performed at different temperatures and reaction times.149
Almost all the results to date have been for batch systems, and most studies have used organic solvents to recover the bio-oil fraction, which is perhaps not necessary in a continuous process. The introduction of organic solvents can increase the bio-oil recovery and will affect the water phase composition. Therefore, batch experiments only give partial insights into a continuous process; nevertheless, studies are useful for obtaining the optimum operating conditions and reaction pathways.
The large volume requirements of biomass conversion would rule out the use of a batch reactor. Thus, continuous operation will be required to make the bio-oil production more economically feasible. Moreover, the heat recovery from a continuous process increases the overall energy efficiency.164 Jazrawi et al. developed a continuous-flow, pilot-scale HTL reactor system for the HTL of microalgae (Chlorella and Spirulina) as a follow-up to earlier research.164 It was anticipated that the maximal bio-oil yield may be obtained at shorter residence times under continuous-flow HTL processing. The investigators demonstrated the successful operation of a continuous-flow, pilot-scale HTL reactor system and provided insight into the processing of microalgae under subcritical conditions. The bio-oil yields reached a maximum of 41.7 wt% for Chlorella processed with a 10 wt% solid concentration at 623 K, 3 min residence time, and 20 MPa. Continuous-flow HTL process provides a basis as well as technical parameters for its further industrialization. With increasing temperature, the oxygen content of the bio-oil decreased and the nitrogen content increased due to conversion of the algal protein fraction.
Recently, Elliott et al. reported a continuous-flow reactor system at relatively low temperature (623 K) and moderate pressure (20 MPa) to produce bio-oil from algae via HTL process.140 A high bio-oil yield was obtained from the continuous HTL of whole algae. An analysis of the bio-oil composition revealed lipid-derived alkane products and heterocyclics derived from other biomass components.
(1) Homogenous catalysts. Homogenous catalysts have received more attention for liquefaction of algae than heterogeneous catalysts.29 The addition of alkali salts has a positive effect on HTL. To date, the most-studied homogeneous catalyst for the HTL of microalgae has been Na2CO3, which has been shown to improve gasification rates, accelerate the water gas shift reaction, and increase overall bio-oil yields.27–29,31–33,57,60,153,155,163,165–169 In addition, the base catalysts raise the pH, inhibiting the dehydration of biomass monomers. Deoxygenation through dehydration (generally catalyzed by acid) instead of decarboxylization (DCO) give unsaturated compounds which easily polymerize to undesired char and tar.36 Thus, alkali suppresses char and tar formation. Notwithstanding their advantages, homogeneous catalysts are more difficult to separate and recover after reaction.29
Minowa et al. carried out the earliest work on homogenously catalyzed liquefaction of microalgae using 5% Na2CO3 solution for the strains on B. braunii and D. tertiolecta.29,163 The addition of Na2CO3 increased the bio-oil yield and energy yield. The HTL of M. viridis with 5% Na2CO3 as the catalyst resulted in the bio-oil yield increasing from 28.0 to 33.0 wt% while the energy yield increasing from 29.4 to 39.5 wt%.33 In addition, the decrease in the oxygen content of the bio-oil was from 24.2 to 19.7 wt%.33 The effect of the catalyst was more pronounced at lower temperature and shorter residence time. Similar results were obtained during the HTL of D. tertiolecta, B. braunii using 5% Na2CO3 as a catalyst; in fact, the obtained bio-oil was nearly equivalent in quality to that of petroleum oil.32,163,169
According to the influence of the content of microalgae on the yields and product distribution, the reported bio-oil yield obtained from HTL microalgae exceeds the lipid content of the algae. The bio-oil yield followed the trend lipids > proteins > carbohydrates.27 Both proteins and lipids were efficiently converted to bio-oil without catalysts, whereas the carbohydrates conversion was enhanced with Na2CO3. The carbohydrate and protein fractions of microalgae in water were converted into bio-oil with efficiencies of nearly 10.0 wt% and 20.0 wt%, respectively.27 The effectiveness of Na2CO3 reportedly depends to a large extent on the reaction temperature. Dote,29 Minowa,28 and Inoue30 found that increasing the liquefaction temperature from 573 to 613 K with Na2CO3 as a catalyst decreased the bio-oil yield. Yang33 and Ross31 reported the opposite effect. These apparently inconsistent effects may be due to the different biochemical compositions of the microalgae, which, as mentioned above, respond differently to the presence of Na2CO3. The lipid content reported by Dote29 and Minowa28 was higher than that for the algae used by Ross,31 supporting this hypothesis.
In some cases the addition of a catalyst may not increase the bio-oil yield but will alter the product distribution. For example, a common catalysts FeS had no effect on the bio-oil yield but significantly altered the proportion of the dominant compound, ethyl hexadecanoate.
The effect of catalyst type on the HTL of algae is another critical issue. For example, the HTL of C. vulgaris and Spirulina using alkalis (KOH and Na2CO3) and organic acids (CH3COOH and HCOOH) as the catalysts were reported by Biller et al.31 The catalysts enhanced the bio-oil yield in the order of Na2CO3 > CH3COOH > KOH > HCOOH. The use of organic acids can improve the flow properties and lower the boiling point of the bio-oil.33 However, the underlying mechanism for the apparent catalytic promotion by Na2CO3 or formic acid during the liquefaction of biological molecule like proteins remains unclear. The common catalyst Na2CO3 can have positive effects on the liquefaction of carbohydrates as well. For this reason, catalyzed HTL of high-carbohydrate microalgae resulted in higher yields with the addition of a carbonate catalyst as compared to the noncatalytic process. On the other hand, the use of alkali with highlipid feedstocks can induce saponification reactions, which leads to soap formation and reduced the bio-oil yield. However, longchain alkanes can be obtained from lipids with the use of Na2CO3. Model protein components investigated were preferably processed in water alone and exhibited the highest yields and energy content at the used conditions. These results suggest that high carbohydrate-containing algae should be processed in alkali, whereas highprotein and highlipid algae are best processed in water alone or in formic acid because these conditions can reduce the boiling point and increase the flow properties.
Another group of catalysts, including alkaline earth metal [Ca3(PO4)2] and transition metal oxide (NiO) were studied for the effect on the bio-oil yield from HTL of the microalga S. platensis for comparisons with alkali metal (Na2CO3).60 Na2CO3 increased the bio-oil yield to 51.6 wt%, which was 29.2 wt% higher than that under noncatalytic conditions. In addition, the presence of NiO and Ca3(PO4)2 increased the yields of gaseous products, while catalytic HTL using Na2CO3 produced lower gaseous yields than noncatalytic conditions. The use of the Ca- and Ni-based catalysts increased the gaseous yields, and decreased the bio-oil formation.
In addition to the alkali Na2CO3 acidic species such as H2SO4 have been used. Zou et al. liquefied the microalga D. tertiolecta at 393–473 K using 0–3.0 wt% H2SO4 as the catalyst in ethyleneglycol (EG).155 A statistical analysis of their data showed that a maximum liquefaction yield of 45.0 wt% could be obtained at the optimized conditions of 2.4 wt% H2SO4 at 443 K for 33 min. The direct liquefaction of Sargassum polycystum C. Agardh in EG with H2SO4 as the catalyst along with microwave-assisted liquefaction resulted in bio-oil yield of 87.7 wt%. In fact, the bio-oil was mainly composed of fatty acid methyl esters and alkanes with chain lengths from C17 to C20.154 When the feedstock was changed into Ulva prolifera, the maximum liquefaction yield of U. prolifera was 84.8 wt% with a HHV of 15.1 MJ kg−1, which was obtained under a microwave power of 600 W using 6.0% H2SO4 as the catalyst via microwave-assisted direct liquefaction.170 The bio-oil was composed of benzene carboxylic acid, diethyl phthalate, long-chain fatty acids (C13 to C18), fatty acid methyl esters, and water.
The use of homogeneous catalysts does not always have a positive effect on the bio-oil yields and properties, especially if the additional cost is considered. Furthermore, the recovery of the homogenous catalysts is a problem.
(2) Heterogeneous catalysts. Heterogeneous catalysts provide a more attractive option than homogeneous catalysts in HTL. Their practical advantage is their separation is accomplished by simple filtration. Moreover, solid catalysts are commonly used in low-temperature water gasification of biomass, and gasification is crucial during HTL because oxygen is removed during this process.161
A large number of studies have been carried out using catalysts to improve bio-oil yield during HTL of microalgae. The choice of catalyst depends on the specific composition of the algal strain. In addition, heterogeneous catalysts tend to undergo coking during HTL process. Moreover, regeneration of the deactivated heterogeneous catalysts was difficult because the catalysts alone cannot be separated from the solid residue. These shortcomings greatly limit their practical application in the HTL of algae, and further works should be performed to overcome these disadvantages.
The most extensive reports on the influence of heterogeneous catalysis on HTL were published by Duan and Savage, who produced crude bio-oils from the microalga Nannochloropsis sp. via HTL in the presence of six heterogeneous catalysts [Pd/C, Pt/C, Ru/C, Ni/SiO2–Al2O3, CoMo/γ-Al2O3 (sulfided), and zeolite].35 The bio-oil yield in the absence of catalysts was 35.0 wt%, but increased to 57.0 wt% when the Pd/C catalyst was used without hydrogen. Ni/SiO2–Al2O3 was the most active catalyst for desulfurization. The bio-oil produced with in the presence of Pd, Pt, Ru and Co–Mo catalysts exhibited a lower viscosity and lighter color than the uncatalyzed or zeolitecatalyzed samples. Meanwhile the presence of Ni, Pt, and Co–Mo decreased the O/C ratio. This suggests that catalytic deoxygenation was promoted.
In another study, NiO was used to catalytic HTL of both single (Spirulina) and mixed algal (from open ponds with wastewater) cultures.158 Unexpectedly, the added NiO decreased bio-oil yields. The maximum bio-oil yield is up to 40.0 wt% in the presence of alumina-supported transition metal catalysts in the temperature range of 573 to 623 K.27 The liquefaction procedure carried out at 623 K gave a bio-oil with a HHV of 39.0 MJ kg−1. Biller et al. investigated three catalysts: an alumina-supported Co/Mo catalyst, an alumina-supported Ni catalyst and an alumina supported Pt catalyst.21 The results indicated that the bio-oil yield from the HTL of C. vulgaris and N. occulta increased slightly with the use of heterogeneous catalysts; however, the increase of HHV was up to 10%. The HTL results of a low-lipid microalga C. pyrenoidosa using heterogeneous catalysts showed that Raney-Ni and HZSM-5 catalysts had no significant effect on the HTL process. H2 as the processing gas slightly improved the bio-oil yield and quality, whereas catalysts have no significant effect.84
Matsui et al. studied the liquefaction of Spirulina with various concentrations of Fe(CO)5–S catalyst.157 Reactions in 1-methylnaphthalene with a small amount of water in CO and Fe(CO)5–S gave conversions greater than 96.0 wt% and total amount of bio-oil, gas and water yields was up to 83.0 wt%.
In summary, catalytic HTL conversion of algae can produce hydrocarbons for liquid fuels and hydrogen/methane-rich product gases. Thus, this field has tremendous potential and a bright outlook. Most recent studies on producing liquid fuels from the HTL of algae have focused on homogeneous catalysis by metal salts or alkali. More work is needed to identify better heterogeneous catalysts for these applications. In particular, the development of nonprecious metal-based catalysts is of particular interest. Finally, active catalytic materials that resist deactivation during HTL are needed.
To address these drawbacks, organic solvents have been studied as alternative media during microalgal liquefaction. Organic solvents used in microalgal liquefaction can dissolve or stabilize the weak polar or even nonpolar intermediates because of their lower dielectric constant compared to water.171 Thus, such solvents can produce higher bio-oil yield. Another advantage of using organic solvents is that more moderate operating conditions can be used.
The HTL of the low-lipid microalga C. pyrenoidosa was processed under sub/supercritical ethanol84 The highest bio-oil yield of algae was 71.3 wt% at 513 K, whereas the highest HHV of the bio-oil was 36.2 MJ kg−1 at 573 K. Supercritical ethanol condition (>513 K) is essential for the conversion of C. pyrenoidosa, and higher temperature facilitates deoxygenation.84
In addition, publication studies have shown that the solvent polarity can have a significant impact on the liquefaction features of microalgae.172 When ethanol as the reaction medium for liquefaction of C. pyrenoidosa, the HHV of the bio-oils produced under different reaction conditions ranged from 27.7 to 36.5 MJ kg−1. The bio-oil yield increased from 9.8 to 64.6 wt% while the solid residue decreased from 60.1 to 11.9 wt% as the temperature was increased from 443 to 623 K. The solvent type is significantly affected the bio-oil product distribution. The dominant components of the bio-oils were fatty acid methyl and ethyl esters when methanol and ethanol were used as solvents, respectively. In contrast, the primary product was hexadecanenitrile when 1,4-dioxane was used as solvent.59 In addition to solvent polarity, operating variables, such as temperature, reaction time, solvent/microalgae ratio, and catalyst type and dosage, also influenced the conversion and yield of the bio-oil. As reported by Matsui et al., reactions in tetralin and hydrogen significantly increased the bio-oil yield as the temperature was increased from 573 to 698 K.157
Additionally, previous studies had proved that the use of a co-solvent with water was advantageous to microalgae HTL on the bio-oil yield and product distribution.173–176 Brennecke et al. found that the solubility of supercritical fluids could be greatly improved by adding a small amount of a second solvent, which is commonly called co-solvent.174 Ethanol, 2-propanol, and methanol are often used as co-solvents for microalgal conversion in HTL.175,176 Significantly higher bio-oil yield can be achieved in co-solvent–water mixture than in water alone as the reaction medium. Investigation of hydrophobic hydration in methanol–water mixtures under supercritical conditions is of a great practical importance for chemical engineering.177,178
Chen et al. reported the production of bio-oil by direct liquefaction of D. tertiolecta with sub/supercritical ethanol–water as the reaction medium at high temperature and pressure.175 The bio-oil and solid residue (SR) yields as well as the conversion, are shown as a function of ethanol content in Fig. 12. The results indicate that using either ethanol or water as the medium is less effective for the conversion or bio-oil yield compared to the ethanol–water mixture. Ethanol and water showed synergistic effects on the liquefaction of D. tertiolecta. Sub/supercritical water can provide ionic, polar nonionic and free radical for the production of bio-oil from microalgae.179
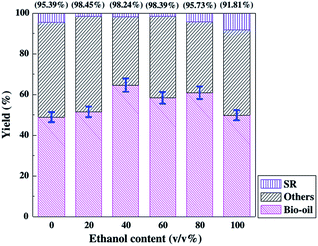 | ||
| Fig. 12 Effect of ethanol content on the bio-oil, others and SR yields for the microalgae liquefaction in ethanol-water cosolvent.175 Copyright 2012 Elsevier. | ||
Other studies have reported the function of ethanol as hydrogen-donors in algae liquefaction for bio-oil production.176 In spite of the interesting synergistic effects afforded by the solvent mixture, the liquefaction of algae with sub/supercritical ethanol–water as the medium is a complex process, and many reactions could occur. The hydrogen-donor function of ethanol cannot be regarded as a simple dehydrogenation of ethanol. Direct evidence of the hydrogen-donor effect could be obtained by the hydrogen isotopic tracer method. Clearly, further research is needed.
(1) Co-liquefaction of microalgae and coal. Coal is an attractive long-term energy source because of its comparatively large reserves. However, its larger sulfur, and metals content calls for the development of clean conversion technologies. Along these lines, direct coal liquefaction is an interesting technology. However, coal liquefaction requires harsh reaction conditions and the requisite hydrogen increases the cost and deters wide-spread adoption. To this end, the co-liquefaction of coal and biomass has gained increasing attention because it takes full advantage of the hydrogen of biomass, which could reduce hydrogen consumption, and result in milder operation compared to direct coal liquefaction.
Earlier studies have investigated the co-liquefaction of microalgae (Chlorella, Spirulina, and Littorale) with coal (Australian Yallourn brown coal and Illinois no. 6 coal) under pressurized H2 in 1-methylnaphthalene at 623–673 K for 60 min with various catalysts.51 Co-liquefaction of Chlorella with Yallourn coal resulted in 99.8 wt% conversion with a 65.5 wt% of hexane-soluble fraction obtained at 673 K using a Fe1−xS catalyst. When Littorale and Spirulina were used, similar results were obtained with an iron-based catalyst. Conversely, the oil yield in the co-liquefaction with Illinois no. 6 coal was close to the additivity of the respective reaction with Fe(CO)5–S, even at S/Fe = 2. Ru3(CO)12was also effective for the co-liquefaction of microalgae with coal.51
Yang et al. reported the co-liquefaction of D. tertiolecta and coal to produce liquid fuel with sub/supercritical water–ethanol as the reaction solvent.180 The optimal conversion and oil yield were 70.6 and 40.3 wt%, respectively. The results showed that an obvious synergetic effect existed between D. tertiolecta and coal, which not only improved the conversion and oil yield but also enhanced the oil quality. The synergetic effect values of conversion and bio-oil yield were 15.7 and 12.5 wt%, respectively, under the optimal reaction conditions.
(2) Co-liquefaction of microalgae and plastic. The rapid growth of plastics use worldwide has led to a concomitant increase in the amounts of plastics waste which is bulky and resistant to degradation. Thus, the conversion of waste plastic to liquid fuels is an intriguing approach especially given its typically high HHV (approximately 40 MJ kg−1) as a result of its high hydrogen and carbon content. The co-processing of waste polymer with biomass has received considerable attention.181 Plastics could provide hydrogen during co-processing with biomass, increase oil production, and improve oil quality because of the high hydrogen content in plastics (approximately 14 wt% for PP and PE). Furthermore, the degradation of polymer could be improved via mixing with biomass.
A study on the co-liquefaction of microalgae (Spirulina) and synthetic polymer (high-density polyethylene, HDPE) in sub/supercritical ethanol showed that the decomposition of Spirulina and HDPE were mutually improved.181 The addition of Spirulina reduced the requisite degradation conditions for HDPE liquefaction and resulted in a high conversion of the HDPE. Synergetic effects were reported for the co-liquefaction of Spirulina and HDPE. For example, with a Spirulina/HDPE feed ratio of 4/6, the oil yield obtained at 613 K increased by 44.8 wt%. The oil from Spirulina/HDPE co-liquefaction had higher carbon and hydrogen content but lower oxygen content, resulting in a HHV of 48.4 MJ kg−1, a level comparable to fossil fuel. Moreover, the chemical compositions of the oil from co-liquefaction of Spirulina/HDPE blends were similar to that from sole HDPE liquefaction, in which aliphatic hydrocarbons dominated.181
In summary, microalgae can have obvious different role when they co-liquefying with different kinds of substances. Microalgae could act as the hydrogen donor in the co-processing of algae with coal because of their higher hydrogen contents. In view of the co-processing with plastics, algae act as the hydrogen receiver because they have less hydrogen than plastics. The obvious synergetic effect exists during co-processing; as a result, both the oil yield and quality have been improved.
The carbohydrates in algae include polysaccharides, celluloses, hemicelluloses and starches. During HTL, carbohydrates are rapidly hydrolyzed to monosaccharides with glucose as one of the main products (Fig. 13).161,182 The glucose is readily converted to fructose, an isomer of glucose. The fructose subsequently undergoes degradation with fragmentation products (e.g., glycolaldehydes and glyceraldehydes). Some short intermediates can form volatile product (e.g., H2, CH4, CO, etc.) and coke via further reaction.
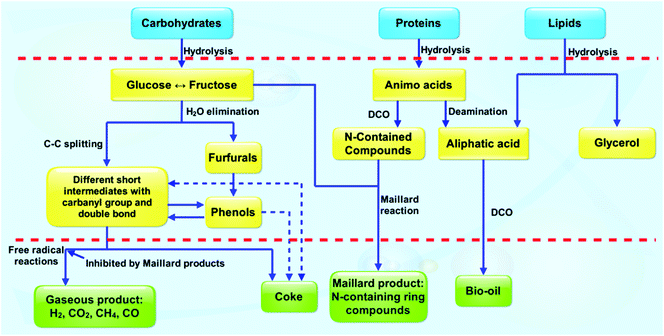 | ||
| Fig. 13 Simplified reaction pathways for HTL of carbohydrates, proteins, and lipids altogether.161 Copyright 2011 Elsevier,182 Copyright 2010 Elsevier. | ||
Lipids mainly consist of fatty acid triglycerides (TAGs), which are nonpolar compounds with aliphatic characteristics. In HTL, TAGs are hydrolyzed to fatty acids and glycerol. The glycerol is subsequently converted to water-soluble soluble compounds. Free fatty acids are relatively stable but partially degrade to produce long-chain hydrocarbons for transportation fuels via DCO (Fig. 13).
Most proteins are composed of linear polymers of amino acids, and they both have structural and metabolic functions. The peptide C–N bond links the amino acids together between the carboxyl and amine groups; this bond will be hydrolyzed under HTL conditions resulting in the production of amino acids (Fig. 13). The amino acids rapidly undergo DCO and deamination, and consequently produce hydrocarbons, amines, aldehydes and acids. Some of these products are the same as those from the hydrolysis of carbohydrate. The interaction between the hydrolysates from carbohydrates and proteins can react with each other to generate N-containing ring compounds. This process is recognized as Maillard reaction, which is confirmed by many other published papers.37,84,161,182–186
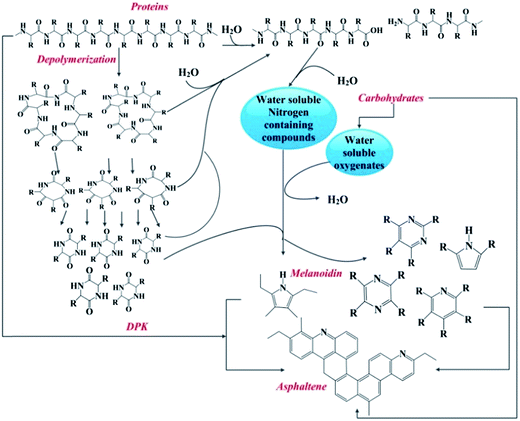
With the aforementioned main chemical pathways identified, the HTL of algae is a complex process that is strongly affected by the feedstock type and the HTL experimental conditions. Knowledge about the composition of HTL bio-oils is important to understand the HTL mechanism. Torri et al. provided the HTL mechanisms based on the chemical compounds in the bio-oils obtained from the HTL of Desmodesmus sp.187 The possible mechanisms are described as follows (Fig. 14).187
 | ||
| Fig. 14 Possible mechanisms for HTL oil formation from protein/carbohydrate macromolecules.187 Copyright 2012 American Chemical Society. | ||
(1) The HTL-derived bio-oil is a mixture of a large number of compounds and macromolecular constituents, ranging from peptides to long-chain hydrocarbons. HTL at relatively low temperature (473–523 K) allows the extraction of the solvent soluble part of the cell constituents. Therefore, lipids, some short-chain algaenans, and some hydrophobic protein fragments end up in the organic solvent phase. However, most proteins and carbohydrates are not converted to water-insoluble products. Below 523 K, HTL is accompanied by a certain degree of thermal degradation, and the extraction of lipids and algaenan is improved to a certain degree. Therefore, the reactivity between proteins and carbohydrates is crucial during HTL. In general, proteins and carbohydrates can interact between themselves and/or with lipids through various ways.
(2) At 573–648 K, proteins and celluloses start to break down, giving diketopiperazines (DKP), amino-acid derivatives, and carbohydrates derivatives (e.g., furans), the products from the cross reaction of those species, and asphaltene-like components. Protein degradation increases the bio-oil yields, but the hydrophobic portion of the protein–carbohydrate derived components increase the nitrogen content of the bio-oil. The main chemical route for the protein conversion is probably through depolymerization. Peptide depolymerization can be described as a progressive cyclization with the formation of gradually smaller cyclic oligo-peptides, with the final product being DKP. Even at 473 K, carbohydrates and proteins can be fragmented into smaller products (e.g., amines and aldehydes), which may be able to form melanoid in-like materials and asphaltene-like materials.
(3) As the reaction proceeds, a strong increase is observed in “pyrolysis-like” products produced from thermal fragmentation of proteins and carbohydrates (e.g., amino acids side chains or 2-methyl-cyclopentenone) and of smaller products from Maillard reactions (e.g., pyrroles), at the expense of peptides and DKP, which are probably converted into amino acids and/or to other by-products.187
As mentioned earlier, introducing organic compounds as the processing solvents in HTL can improve the quality of bio-oil. The HTL mechanism of microalgae in ethanol is different from that in water. Based on GC-MS results and published reports, Zhang et al. provided a potential HTL mechanism of algae (Fig. 15):84 (1) carbohydrates, proteins, and lipids first break down to their corresponding monomers, such as glucose, xylose, phenols, amino acids, and fatty acids under HTL conditions. These monomers further decompose, and then to form various types of intermediates. (2) The monomers and their intermediates undergo a series of reactions. As illustrated in Fig. 15, the amino acids undergo DCO and deamination reactions to form the corresponding amines and keto acids, respectively. The keto acids undergo DCO to form ketones, which are abundant in the liquid products. The fatty acids undergo DCO to form aliphatic hydrocarbons or react with ethanol through esterification to form fatty acid esters. The fatty acids also react with amines or ammonia through acylation to form amides. The monosaccharides react with amino acids through Maillard chemistry to form melanoidin (nitrogenous polymers) and solid products. The monosaccharides further decompose to form small acids and furfural derivatives. The furfural derivatives and phenols undergo repolymerization to form large molecular components and solid products.84 The competitive reaction of the carboxyl group and ethanol to form esters, which are more stable under HTL conditions, suppress DCO and which results in a decrease in the volatile product yield.
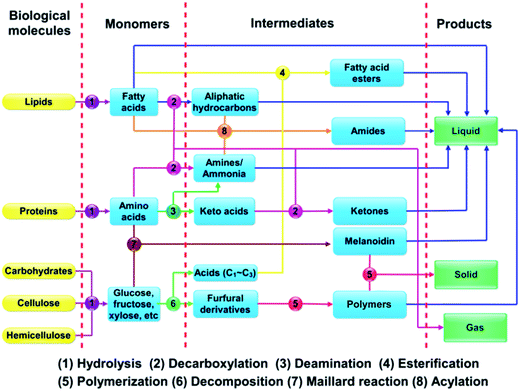 | ||
| Fig. 15 Potential reaction mechanism of HTL for algae.84 Copyright 2013 Elsevier. | ||
Water plays a crucial role during the HTL of algae. Of particular importance is hydrolysis which converts proteins, carbohydrates, and lipids into a variety of components. To this end, the use of a cosolvent with water has attracted considerable attention because of the possible redirection reaction pathways. Chen et al. presented a plausible reaction mechanism on bio-oil preparation from D. tertiolecta with sub/supercritical ethanol-water as the reaction medium (Fig. 16).175 Sub/supercritical ethanol–water is a weak acid. The liquefaction of proteins, carbohydrates, and lipids in D. tertiolecta is acid-catalyzed. Under acidic conditions, proteins first form a long peptide chain, which is then hydrolyzed to form amino acids. The amino acids undergo cracking, condensation, DCO, deamination, etc. to form liquefied product. Carbohydrates undergo dehydration to form monosaccharides, a part of which may then react with ethanol to form the ether that exists in the solid residues. Most monosaccharides may further react to generate carboxylic acid or other organic compounds that undergo the acid-catalyzed process. Lipids undergo dehydration to form carboxylic acid and glycerol. A competitive reaction with carboxylic acid occurs between ammonia and ethanol to form amides and esters during HTL. With sufficient ethanol present, amides react with ethanol. The presence of ethanol significantly influences the composition of liquefied products. The carboxylic acid can then react with ethanol via alcoholysis to form carboxylic acid esters and undergo ammonolysis with ammonia to form amides. The amides can also undergo alcoholysis to generate carboxylic acid esters when the concentration of ethanol is high. Ammonia, which is produced during the acid-catalyzed decomposition of proteins, may be used as an ammonolysis reagent in direct liquefaction.175
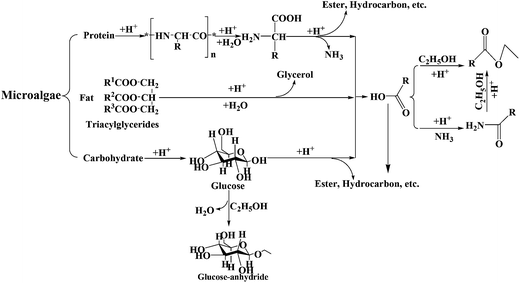 | ||
| Fig. 16 HTL mechanisms of the main components of microalgae in sub/supercritical ethanol–water cosolvent.175 Copyright 2012 Elsevier. | ||
In summary, macromolecules in the microalgae first hydrolyze into small fragments (fatty acids, amino acids, and glucose), which are then converted into even lower molecular weight compounds. Particularly, amino acids undergo DCO and deamination to produce hydrocarbons, amines, aldehydes, and acids. The generated compounds (intermediates) are unstable and rearrange into larger compounds via condensation, cyclization, and polymerization. Although many researchers have studied the mechanism of HTL, understanding the detailed interactions during the HTL of microalgae and their kinetics warrant further research.
Microalgal biomass, which has potential to serve as a renewable source of energy, is relatively new and unexplored. HTL will most likely find its place in an energy system where algae are used because of its safe and cheap reactant (water). Any commercial application of algae HTL would require the use of a continuous process where heat recovery can be incorporated in order to be sufficiently energy efficient. To achieve high yields of high quality bio-oil, the use of catalysts and potentially co-solvent is needed. Further research is needed to identify these catalysts and to understand the reaction pathways and to quantify the HTL kinetics. The following are recommended key factors that will have to be addressed pertaining to HTL microalgae:
• Catalyst. Catalytic HTL is one of the most ideal methods of microalgal liquefaction, and a suitable catalyst can increase biofuel yield and improve quality of bio-oil as well as. However, homogenous catalysts present a serious problem on the recovery of the catalysts, and the hydrothermal stability of heterogeneous catalysts must be taken into account when water as the reaction medium during HTL microalgae process. Further studies on more catalysts are necessary to identify supports and active materials that can better resist deactivation in HTL.
• Mild reaction conditions. HTL with sole water as the medium has potential drawbacks including relatively harsh with rather high temperatures (523–623 K) and high pressures (10–20 MPa). To solve these problems, organic solvents or organic solvents–water mixture instead of water have been applied during microalgal HTL. Replacing water with organic solvent or mixture as the reaction medium for HTL is theoretically feasible. The main advantage of using organic solvents or mixture is that more moderate operating conditions can be obtained. Organic solvents that have been used for microalgae liquefaction include alcohol, dioxane, tetralin, 1-methylnaphthalene, toluene and chloroform, etc.
• Development of pilot-scale plant. Process development work related to continuous operation and scale up has been reported. Process development, design, and optimization are facilitated by the availability of mathematical models that faithfully describe the process chemistry. The development of economic pilot-scale plant of HTL microalgae to produce bio-oil is one trend of HTL development.
• Reaction mechanisms and kinetics. Full knowledge of the mechanisms involved in the HTL process is critical. A systemic approach in determining the effects of various feedstock components and different reaction conditions on bio-oil yield and quality is needed because numerous algae species could be used as feedstock and different reaction conditions could be carried out.
4.3 Comparison of pyrolysis and HTL of algae
As mentioned above, HTL is a low-temperature (523–623 K) and high-pressure (5–20 MPa) process particularly suited for high-moisture feedstocks. In contrast, pyrolysis is accomplished at moderate to high temperatures (673–873 K) and atmospheric pressure but requires feedstock drying. Considerable attention has been given to HTL and pyrolysis for algal conversion. However, evaluating these two competing methods is complicated by the myriad of different algae strains. For this reason, studies that have directly compared the two methods using the same feedstock can provide valuable information.One such study was conducted by Jena and Das who compared the liquefaction and pyrolysis of S. platensis.61 The conversion was performed with a slurry containing 20.0 wt% of algal biomass and a reaction time of 60 min. Detailed comparison results are listed in Table 5. The energy consumption ratio (ECR) of HTL was found to be 0.70, indicating that this process was a net energy producer. In contrast, the ECR value of pyrolysis suggested that the pyrolysis consumed more energy than what could be produced from algal feedstock. Moreover, the bio-oil obtained from HTL had a higher energy density and superior fuel properties such as thermal and storage stability, compared to that obtained from pyrolysis.188
| Liquefaction | Slow pyrolysis | ||
|---|---|---|---|
| Reaction temperature/K | 623 | 623 | 773 |
| Conversion/wt% | 93.0 | 60.0–72.0 | — |
| Bio-oil yield/wt% | 40.7 | 23.8 | 28.5 |
| HHV/MJ kg−1 | 34.2 | 29.3 | 33.6 |
| Energy recovery from the original algae/% | 67.9 | 33.9 | 46.7 |
| ECR | 0.7 | 2.11 | 1.56 |
The compositions of bio-oil from HTL and pyrolysis have notable differences. Bio-oils generated from pyrolysis, especially at 623 K, have higher percentages of nitrogenous compounds and aromatic heterocycles compared to those generated from HTL. Bio-oils generated from HTL are easier to upgrade than those from pyrolysis because of the higher abundance of straight-chain compounds than that of the former. FT-IR spectra of the bio-oil samples obtained from HTL and pyrolysis of microalgae are shown in Fig. 17.61 A distinct band at approximately 3300 cm−1 for pyrolytic bio-oils corresponds to N–H functional groups and represents a higher abundance of nitrogenous compounds than that for HTL bio-oils. Lower peaks in bio-oil from HTL at 1670 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) represent less abundance of carboxylic acids, esters, or aryl ketones than that in bio-oil from pyrolysis.
O) represent less abundance of carboxylic acids, esters, or aryl ketones than that in bio-oil from pyrolysis.
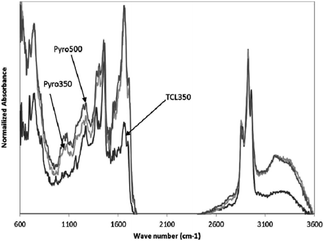 | ||
| Fig. 17 Infrared spectra of bio-oil samples obtained from HTL and pyrolysis of microalgae.61 Copyright 2011 American Chemical Society. | ||
Bio-oil from HTL had higher amount of inorganic elements than that from pyrolysis. This result can be attributed to HTL being a high-pressure process that results in more intense reactions compared to pyrolysis. Greater amounts of inorganic elements could have leached from solids ending up in the liquids/bio-oil fraction. In comparison, the bio-oil vapor from pyrolysis was collected in a set of condensers, leaving the solids inside the main reactor, as the reaction further proceeded.
In a second study, Vardon et al. converted Scenedesmus (raw and defatted) and Spirulina to bio-oil by HTL (573 K and 10–12 MPa) and slow pyrolysis (heated to 723 K at a rate of 50 K min−1), and then compared the produced bio-oils.86 Both the two conversion routes produced energy-dense of bio-oil (35–37 MJ kg−1) similar to shale oil (41 MJ kg−1). However, bio-oil yields (24–45 wt%) and physicochemical characteristics were greatly influenced by conversion route and algal strains. Notable differences were observed in the mean bio-oil molecular weight (pyrolysis: 280–360 Da; HTL: 700–1330 Da) and the percentage of low-boiling compounds (bp < 673 K) (pyrolysis: 62–66 wt%; HTL: 45–54 wt%). HTL and slow pyrolysis of algae produced bio-oils with similar HHV, heteroatom content, and functionality. Nevertheless, pyrolytic bio-oil displayed a significantly higher percentage of cyclic oxygenates (16–24 wt%) compared to HTL bio-oil (8–12 wt%) in the form of phenolic compounds.
The energy efficiency of the two conversion routes was also discussed. Analysis of ECR also indicated that HTL is more favorable (ECR 0.4–0.6) than pyrolysis (ECR 0.9–1.2) for processing wet algal biomass (80% moisture content) because the latter requires water volatilization. However, pyrolysis is energetically favorable if the starting algal biomass has low moisture content (Fig. 18).86
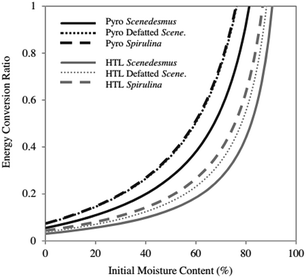 | ||
| Fig. 18 ECR for HTL and pyrolysis of algal biomass at varying initial moisture contents.86 Copyright 2012 Elsevier. | ||
Finally, HTL and pyrolysis have different dewater requirement and schedule. HTL is favorable for wet algal conversion because of its integration with wet microalgae slurry (10–20% solids), as opposed to pyrolysis, which requires dried microalgae (80% solids). The dewatering requirements to achieve the percent solids required for the HTL necessitates the use of bio-flocculation, dissolved air filtration and centrifugation for water removal. The pyrolysis pathway requires the remaining water to be removed by using thermal methods. Drying of microalgae requires substantial energy, accounting for nearly half of the overall net energy ratio (NER) for the pyrolysis pathway modeled at the industrial-scale.189
A similar conclusion was reported by Sawayama.185 According to their calculations, the energy required for algae liquefaction is only 6.7 MJ kg−1 of the bio-oil produced. Therefore, HTL is preferred over pyrolysis for the conversion of algae to bio-oil because of its energetic and economic advantages.
Although HTL may be more attractive than pyrolysis with respect to energy efficiency, not all studies agree that HTL has a positive ECR. Biller and Ross employed HTL on four algal species (C. vulgaris, Spirulina, Nannochloropsis oc. and P. cruentum) and indicated that only the HTL of C. vulgaris without catalyst produced an ECR of 0.8. The ECRs of all other species were all equal to or larger than 1.0, suggesting that more energy is needed for liquefaction than what can be gained from the bio-oil.27 Therefore, algal conversion to bio-oil by HTL must be performed with caution.
5. Upgrading of crude bio-oil from algae
As we have discussed, bio-oil derived from algae via the thermochemical conversion processes of pyrolysis and HTL has a higher oxygen content, lower stability and lower HHV than petroleum. This is in spite of the substantial advances made in catalytic pyrolysis and HTL towards the enhancement of bio-oil yield and quality. The upshot is that the bio-oil requires further upgrading to put in on par with conventional petroleum feedstock for subsequent refining into transportation fuel. As discussed above, generally HTL bio-oil has lower oxygen and nitrogen content than bio-oil obtained by pyrolysis.31 The ultimate aim of upgrading is to improve the quality of bio-oil by decreasing the fraction of organic acids, aldehydes, and other reactive compounds because they increase corrosiveness and acidity. Moreover, compared to bio-oil derived from lignocellulosic biomass, microalgal bio-oil contains significant amounts of nitrogen rooted from protein in microalgae, which is undesirable in the final product.Because of the high diversity of compounds in the bio-oil, the upgrading of bio-oil is a complex reaction network; representive reactions include cracking, decarbonylation, DCO, hydrocracking, hydrodeoxygenation (HDO), and hydrogenation.190–194 These are discussed next.
Catalytic cracking with various zeolite catalysts is an attractive method for upgrading triglycerides to produce fuels.195–198 Cracking does not require H2, which is a significant advantage over deoxygenation using hydrotreating catalysts. However, cracking has the disadvantage of coking of the catalyst, which therefore requires regeneration to maintain activity.
DCO and decarboxylation yield hydrocarbon chains with one carbon atom less as compared to the reactant, that is to say, carbon chain length is reduced, which is generally undesirable for fuels. Decarbonylation yields olefins, and DCO produces paraffins. By contrast, the HDO route selectively cleaves C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, while the C–C bonds remain intact. Furthermore, the HDO reaction eliminates oxygen by producing H2O instead of CO2, rendering it more environmentally friendly than DCO, and attract more attentions.
O bonds, while the C–C bonds remain intact. Furthermore, the HDO reaction eliminates oxygen by producing H2O instead of CO2, rendering it more environmentally friendly than DCO, and attract more attentions.
It is worth mentioning, in order to improve fuel combustion performance it may be desirable to retain some oxygen in the fuel. These methods include esterification and selective-hydrogenation.199 Wan et al. reported one-step hydrogenation–esterification of aldehyde and acid to ester over bifunctional Pt catalysts.200 However, production of oxygenated biofuel only be reported in upgrading of bio-oil derived from lignocellulosic biomass, and there are no published data for algae-base bio-oil, it may be important topic worth studying in the future.
5.1 Upgrading of model compounds
Algal-derived bio-oil contains unsaturated and saturated long-chain fatty acids (C16–C18), so it is of interest to understand the relevant upgrading chemistries for these species. Here we report on selected studies.In recent work, the mechanisms for decarbonylation and DCO were investigated using palmitic or oleic acids as model compounds on different catalysts.201–205 The main products from palmitic acid were C8–C15 n-alkanes, with pentadecane the alkane with the highest yield. Hydrogenation of the double bond in oleic acid was faster than its DCO to form C17 hydrocarbons.
Noble metal-based catalysts have high activity and have been extensively investigated. Na et al. investigated the deoxygenation of bio-oil obtained by pyrolysis of microalgae for the production of hydrocarbon fuel by metal-supported catalysts.206 The distribution of the products depends on the reaction conditions and catalyst used.207 To reduce the cost, tungsten-based catalysts were explored.208 Both tungsten oxide and tungsten carbide based catalysts allow an upgrading of the feed to higher-value products. The activated carbon is promising and inexpensive catalytic materials for converting fatty acids to alkanes.209,210
Upgrading via hydrogenation is a promising route and another research focus.197 In a series of related works, Lercher and coworkers211–213 reported that the extracted microalgae oil from algae cell is found to be triglycerides predominant. The upgrading process begins with the hydrogenolysis of the triglycerides to produce fatty acids. The research showed that microalgal oil can be nearly quantitatively hydrodeoxygenated to alkanes by a cascade of reactions on bifunctional catalysts containing Ni and an acidic zeolite (HZSM-5 and HBeta).211 The reaction pathway proceeds through an initial hydrogenolysis of triglyceride leading to fatty acid and propane. The subsequent hydrogenation of the carboxylic group of fatty acid leads to the corresponding aldehyde; for example, octadecanal (rate-determining step), followed by either decarbonylation of octadecanal to n-heptadecane and carbon monoxide (minor route) or hydrogenation of octadecanal to 1-octadecanol (major route). Subsequently, the produced 1-octadecanol undergoes sequential acid-catalyzed dehydration and metal-catalyzed hydrogenation leading to the final n-octadecane. The overall reaction pathway proposed for microalgae oil transformation is shown in Fig. 19A.211 In summary, Ni catalyzes efficiently the hydrogenolysis of the fatty acid ester, the decarbonylation of aldehyde intermediates, and the hydrogenation of –COOH, –CHO, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in reactants and intermediates, and the acid function catalyzes the dehydration of alcohol intermediates.
C double bonds in reactants and intermediates, and the acid function catalyzes the dehydration of alcohol intermediates.
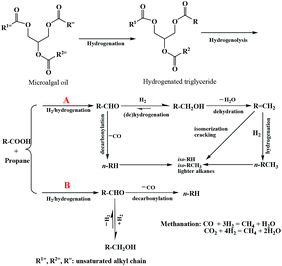 | ||
| Fig. 19 Proposed reaction pathway for the transformation of microalgal oil to alkanes over different catalysts (A) bifunctional Ni/HBeta catalysts.211 Copyright 2009 Wiley; (B) Ni/ZrO2 catalyst.213 Copyright 2012 Wiley. | ||
It was shown that the HDO rate for palmitic acid was greater on Ni/ZrO2 than on Ni/SiO2 or Ni/Al2O3 but was slower than that on H-zeolite-supported Ni.213 In the absence of H2, ketonization catalyzed by ZrO2 is the dominant reaction (Fig. 19B). Pd/C favors direct DCO (-CO2), whereas Pt/C and Raney Ni favor decarbonylation pathway (–CO). The deoxygenation rate of palmitic acid decreases in the sequence of r(Pt black) ≈ r(Pd black) > r(Raney Ni) without H2. The reaction mechanism is the same for either noble mental or transition metal. The development of more affordable catalysts with similar performance and durability is of great interest from an industrial standpoint because noble metals are costly.
5.2 Upgrading of real bio-oil from HTL microalgae
Bio-oils produced from microalgae via HTL are formed in an aqueous environment. From the view of engineering, oil upgrading under the same environment may be more advantageous. Thus, bio-oil treatment in sub- or supercritical aqueous environment may be an effective approach for oil upgrading.Duan published a series of related works.35,214–216 Table 6 provides a summary of Duan's contribution to bio-oil research. The combination of high temperature and H2 increase the HHV of bio-oil. In addition, the performance of carbon-based catalysts is superior to that of other catalysts.
In Duan's work, the upgraded bio-oil had better properties after processing with Pt/C catalyst and high-pressure H2 in SCW. The bio-oil had a HHV of 43 MJ kg−1, and the total acid number of the upgraded oil (25) was considerably lower than that of original feed (256). Finally, the upgraded bio-oil had a very high content of hydrocarbon molecules, including alkanes and aromatic compounds. Overall, the properties of the upgraded oil obtained from the catalytic treatment in SCW are similar to those of hydrocarbon fuels derived from petroleum. This work shows that the crude bio-oil from the HTL of microalgae can be effectively upgraded in SCW in the presence of a Pt/C catalyst.
On the other hand, molecular breakdown of the crude and upgraded oils are clearly different. The chromatogram for the crude bio-oil shows minimal material eluting prior to 40 min. By contrast, the upgraded oil shows some large peaks at retention times shorter than 12 min and many regularly spaced peaks, which correspond to a series of n-alkanes starting at about C9. It indicated the catalytic hydrothermal upgrading process produced oil with more low-boiling species.
During upgrading of crude algal bio-oil in SCW, different types of reactions occur simultaneously. In addition to hydrogenation, hydration and/or oxygenation reaction also occur, which involve water in the upgrading chemistry, leading to the incorporation of H and O atoms into the upgraded oil.
The catalyst must endure erosion from sub/supercritical system, and the effect of H2O on bio-oil treated, which was not found in the published papers, should be considered. Most results were obtained on a small scale, and product characterization has mainly been restricted to GC-MS. Bio-oils from sub/supercritical system treatment are still far away from direct use as transportation fuel because the product oil still contained some oxygenated compounds and nitrogen-containing compounds. Therefore, additional treatment and process optimization is needed.
6. Conclusions and perspectives
In recent years, the production of renewable fuel obtained from microalgae has attracted considerable attention because of algal fast growth rate, minimal competition with food crops, and other factors. Therefore, microalgal-based biofuels have a paramount role to play in combating energy shortage, global warming and climate changes. Thus, there is tremendous potential for this field and the outlook is bright.Nevertheless, research for the production of biofuels from microalgae is in the early stages, we are still on some way from realizing the potential to produce commercially viable microalgal biofuels at a large scale. Sustained, in-depth research is needed to accelerate the practical use of microalgae as energy feedstock, and enhancement of bio-oil production yield and energy efficiency of the process is our overall goal.
In summary, thermochemical conversion of microalgal feedstock is still in the developmental stage, and challenges in the coming years include the following:
• Feedstock provide and algal cultivation
The main hurdle of algae-based biofuels to be solved is the cost gap, since the technologies (including cultivation, harvesting and conversion) accessible today cannot make microalgal biofuels production economical. The cost of the infrastructure facilities and the energy required for microalgae cultivation and harvesting are high, for example, the drying operation of the microalgal biomass consume intense input energy.
The growth rate of algae is fast in comparison to other plants, but in the context of the overall economics higher rates will be needed to reduce the aforementioned land requirements. Genetic engineering of algal strains with high growth rate and energy content is critical. In addition, it appears that choice of an appropriate algal strain and cultivation conditions possibly incorporating flue gas as a source of carbon
• Life cycle analysis
Microalgae obviously require CO2 for growth, which raises the importance of coupling their growth with CO2-producing facilities, such as cement and coal-fired power plants. For example, the use of CO2 absorption columns with algae growth and harvesting comprises a synergistic approach that provides integrated solutions, which could bring costs of CO2 capture and utilization down.
Production volume, land footprint and water requirement. The sheer volume of transportation fuel consumption worldwide is daunting. For example, the U. S. alone consumes about 19 million barrels of petroleum products per day. Of that total, about two-thirds are used for transportation. Based on a conservative estimate for growth rate of 81 g dry algae per m2 day, and assuming that 25% of the intrinsic energy content is utilized towards bio-oil, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2 (6.2 million acres) of land area, 8.466 × 108 m3 saline water and 7.914 × 109 m3 fresh water would be needed to produce the equivalent of 1
000 km2 (6.2 million acres) of land area, 8.466 × 108 m3 saline water and 7.914 × 109 m3 fresh water would be needed to produce the equivalent of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 barrels of oil equivalent per day. Thus, a vast land area and water would be needed.
000 barrels of oil equivalent per day. Thus, a vast land area and water would be needed.
• Process coupling and co-processing
Due to the high cost, algal cultivation for biofuel production alone is hard to achieve cost effectiveness and a positive energy balance. One possible solution to reduce the algae production cost and process energy cost is to combine the algal cultivation with current wastewater treatment process.
In addition, co-processing of microalgae with other feedstocks could also help reduce the overall cost, co-process with waste plastics is such a good example. Waste plastics caused serious environment pollution, which can be regarded as “white pollution”. Addition of a fraction of the waste plastics during the thermochemical conversion of microalgae can obtain higher yield and higher oil quality due to role of waste plastic as hydrogen source and presence of a synergistic effect in co-process.
• Specific issue in conversion process
a Catalyst
Catalysts are crucial in the production of bio-oil from algae. The selection of proper catalysts for the conversion of algae to desired products is a complex process. Promising catalyst candidates must be identified from vast numbers of trial compounds to provide useful information about active sites, optimized structures and composition, and possible synthesis routes. The majority of the work to date on producing liquid fuels from algal HTL has focused on homogeneous catalysis by metal salts or alkali. The more recent studies, however, are beginning to examine heterogeneous catalysts due to advantages in separation and selectivity of the catalyst. In particular, the development of non-precious metal based catalysts would provide a major advance. Finally, there is a need for more catalyst development work to identify supports and active components that better resist deactivation in hot compressed water or supercritical water.b Reaction mechanisms and kinetics
Understanding the mechanisms of algal thermochemical conversions, especially catalysis mechanism, is the critical issue for improving conversion processes and guide the experimental research, and further studies could provide insights into the catalysis mechanism, especially concerning the catalyst deactivation.In addition, process kinetics is extremely to optimized algal conversion reaction and design the pilot-scale reactor in the next step. The effects of various feedstock components and operating parameters on the yield and quality of bio-oil must be further investigated to identify the optimal processing conditions because numerous algae species could be used as feedstock and different reaction condition could be conducted.
c Continuous pilot-scale reactor
Development of pilot-scale plant is needed to accelerate algal conversion process profitability and economic feasibility. However, most of the algal conversion reactions reported have been on a small scale (less than 10 g algae), and only very few attempted algal pyrolysis and HTL have been carried out in continuous reactor systems. Designing a large reactor is very difficult because the mechanisms involves complex process reactions are not yet clearly understood. Finally, during scale up of the HTL process, technical difficulties arise when pumping algae slurry into high pressure reactors.d Product characterization
Understanding the chemical composition of bio-oil is required to evaluating conversion process and optimizing the upgrading of crude-oil. However, the small scale of most algal conversion reactions results in too little sample being obtained to distill the liquid product. Thus product characterization has been limited to GC-MS and elemental analysis for the liquids. When larger amount of sample be obtained, other analytic method, such as 1H NMR spectra, can be conducted, 1H NMR can provide more detailed information about the composition of the total liquid product.e Computational modelling
The computational modelling for algal thermochemical conversion so far is still very limited due to lack of accumulated knowledge in this relatively new field, most related work has been conducted on simplified model systems. More simulation research in this field is expected to be performed in the future, and direct simulation of real algal systems is highly desired. To this end, algae and its conversion process should be understood more deeply and efficient new methodologies, which are capable of simulating large real algal conversion systems, should be developed.Acknowledgements
This study was supported financially by National Natural Science Foundation of China (no. 21376140 and no. 21176142), Program for New Century Excellent Talents in University (no. NCET-12-0308), Independent Research Programs of Tsinghua University (no. 20111081067), and Science and PetroChina Innovation Foundation (no. 2010D50060406) National Key Technology R&D Program (no. 2011BAD14B01).References
- T. M. Yeh, J. G. Dickinson, A. Franck, S. Linic, L. T. J. Thompson and P. E. Savage, J. Chem. Technol. Biotechnol., 2013, 88, 13–24 CrossRef CAS.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- L. R. Lynd, J. H. Cushman, R. J. Nichols and C. E. Wyman, Science, 1991, 251, 1318 CAS.
- Y. Q. Pu, S. L. Cao and A. J. Ragauskas, Energy Environ. Sci., 2011, 4, 3154–3166 CAS.
- C. Zhao, T. Brück and J. A. Lercher, Green Chem., 2013, 15, 1720–1739 RSC.
- W. Wang, S. P. Wang, X. B. Ma and J. L. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- C. Y. Chen, X. Q. Zhao, H. W. Yen, S. H. Ho, C. L. Cheng, D. J. Lee, F. W. Bai and J. S. Chang, Biochem. Eng. J., 2013, 78, 1–10 CrossRef CAS PubMed.
- A. L. Ahmad, N. H. M. Yasin, C. J. C. Derek and J. K. Lim, Renewable Sustainable Energy Rev., 2011, 15, 584–593 CrossRef CAS PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC.
- T. T. Li, Y. B. Zheng, L. Yu and S. L. Chen, Bioresour. Technol., 2013, 131, 60–67 CrossRef CAS PubMed.
- R. H. Wijffels and M. J. Barbosa, Science, 2010, 239, 796–799 CrossRef PubMed.
- B. H. Gebreslassie, R. Waymire and F. Q. You, AIChE J., 2013, 59, 1599–1621 CrossRef CAS.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS PubMed.
- R. S. Ruan, Z. Y. Du, Y. C. Li, X. Q. Wang, Y. Q. Wan, Q. Chen, C. G. Wang, X. Y. Lin, Y. H. Liu and P. Chen, Bioresour. Technol., 2011, 102, 4890–4896 CrossRef PubMed.
- P. M. Schenk, S. R. T. Hall, E. Stephens, U. C. Marx, J. H. Mussgnug, C. Posten, O. Kruse and B. Hankamer, BioEnergy Res., 2008, 1, 20–43 CrossRef PubMed.
- P. Biller, R. Riley and A. B. Ross, Bioresour. Technol., 2011, 102, 4841–4488 CrossRef CAS PubMed.
- L. Gouveia and A. C. Oliveira, J. Ind. Microbiol. Biotechnol., 2009, 36, 269–274 CrossRef CAS PubMed.
- H. M. Oh, J. Y. Lee, C. Yoo, S. Y. Jun and C. Y. Ahn, Bioresour. Technol., 2010, 101, S75–S77 CrossRef PubMed.
- J. E. Duffy, W. A. Canuel, W. Adey and J. P. Swaddle, Science, 2009, 326, 1345 CrossRef CAS PubMed.
- P. J. L. B. Williams, Nature, 2007, 450, 478 CrossRef CAS PubMed.
- G. W. Roberts, M. O. P. Fortier, B. S. M. Sturm and S. M. S. Williams, Energy Fuels, 2013, 27, 857–867 CrossRef CAS.
- M. Balat, M. Balat, E. Kirtay and H. Bala, Energy Convers. Manage., 2009, 50, 3158–3168 CrossRef CAS PubMed.
- A. Gutierrez, R. K. Kaila, M. L. Honkela, R. Slioor and A. O. I. Krause, Catal. Today, 2009, 147, 239–246 CrossRef CAS PubMed.
- D. M. Alonso, J. Q. Bond, J. C. S. Ruiz and J. A. Dumesic, Green Chem., 2010, 12, 992–999 RSC.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- G. W. Huber, J. W. Shabaker and J. A. Dumesic, Science, 2003, 300, 2075–2077 CrossRef CAS PubMed.
- P. Biller and A. B. Ross, Bioresour. Technol., 2010, 102, 215–225 CrossRef PubMed.
- T. Minowa, S. Yokoyama, M. Kishimoto and T. Okakura, Fuel, 1995, 74, 1735–1738 CrossRef CAS.
- Y. Dote, S. Sawayama, S. Inoue, T. Minowa and S. Yokoyama, Fuel, 1994, 73, 1855–1857 CrossRef CAS.
- S. Inoue, Y. Dote, S. Sawayama, T. Minowa, T. Ogi and S. Yokoyama, Biomass Bioenergy, 1994, 6, 269–274 CrossRef CAS.
- A. B. Ross, P. Biller, M. Kubacki, H. Li, A. L. Langton and J. Jones, Fuel, 2010, 89, 2234–2243 CrossRef CAS PubMed.
- S. P. Zuo, Y. L. Wu, M. D. Yang, I. Kaleem, C. Li and J. M. Tong, Energy, 2010, 35, 5406–5411 CrossRef PubMed.
- Y. Yang, C. Feng, Y. Inamori and T. Maekawa, Resour., Conserv. Recycl., 2004, 43, 21–33 CrossRef PubMed.
- L. Li, E. Coppola, J. Rine, J. L. Miller and D. Walker, Energy Fuels, 2010, 24, 1305–1315 CrossRef CAS.
- P. G. Duan and P. E. Savage, Ind. Eng. Chem. Res., 2011, 50, 52–61 CrossRef CAS.
- M. C. Chow, W. R. Jackson, A. L. Chaffee and M. Marshall, Energy Fuels, 2013, 27, 1926–1950 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Froling, J. M. J. Antal and J. W. Tester, Energy Environ. Sci., 2008, 1, 32–65 CAS.
- S. N. Xiu and A. Shahbazi, Renewable Sustainable Energy Rev., 2012, 16, 4406–4414 CrossRef CAS PubMed.
- F. Metting, J. Ind. Microbiol. Biotechnol., 1996, 17, 477–489 CrossRef CAS.
- L. S. Silva, D. L. González, A. M. G. Minguillan and J. L. Valverde, Bioresour. Technol., 2013, 130, 321–331 CrossRef PubMed.
- S. H. Ho, C. Y. Chen, D. J. Lee and J. S. Chang, Biotechnol. Adv., 2011, 29, 189–198 CrossRef CAS PubMed.
- G. Yu, Y. H. Zhang, L. Schideman, T. Funk and Z. C. Wang, Energy Environ. Sci., 2011, 4, 4587–4595 CAS.
- E. W. Becker, Biotechnol. Adv., 2007, 25, 207–210 CrossRef CAS PubMed.
- W. M. Peng, Q. Y. Wu, P. G. Tu and N. M. Zhao, Bioresour. Technol., 2001, 80, 1–7 CrossRef CAS.
- A. M. Rizzo, M. Prussi, L. Bettucci, I. M. Libelli and D. Chiaramonti, Appl. Energy, 2013, 102, 24–31 CrossRef CAS PubMed.
- V. Budarin, A. B. Ross, P. Biller, R. Riley, J. H. Clark, J. M. Jones, D. J. Gilmour and W. Zimmerman, Green Chem., 2012, 14, 3251–3254 RSC.
- K. G. Wang and R. C. Brown, Green Chem., 2013, 15, 675–681 RSC.
- B. Maddi, S. Viamajala and S. Varanasi, Bioresour. Technol., 2011, 102, 11018–11026 CrossRef CAS PubMed.
- G. A. Laura, T. Cristian, S. Chiara, V. S. Jaapjan, F. Daniele, S. R. A. Kersten and D. W. F. Brilman, Energy Fuels, 2012, 26, 642–657 CrossRef.
- A. Demirbas, Energy Convers. Manage., 2010, 51, 2738–2749 CrossRef CAS PubMed.
- N. Ikenaga, C. Ueda, T. Matsui, M. Ohtsuki and T. Suzuki, Energy Fuels, 2001, 15, 350–355 CrossRef CAS.
- X. L. Miao, Q. Y. Wu and C. Y. Yang, J. Anal. Appl. Pyrolysis, 2004, 71, 855–863 CrossRef CAS PubMed.
- S. S. Toor, H. Reddy, S. G. Deng, J. Hoffmann, D. Spangsmark, L. B. Madsen, J. B. H. Nielsen and L. A. Rosendahl, Bioresour. Technol., 2013, 131, 413–419 CrossRef CAS PubMed.
- T. M. Brown, P. G. Duan and P. E. Savage, Energy Fuels, 2010, 24, 3639–3646 CrossRef CAS.
- Z. Y. Du, M. Mohr, X. C. Ma, Y. L. Cheng, X. Y. Lin, Y. H. Liu, W. G. Zhou, P. Chen and R. S. Ruan, Bioresour. Technol., 2012, 120, 13–18 CrossRef CAS PubMed.
- A. Campanella, R. Muncrief, M. P. Harold, D. C. Griffith, N. M. Whitton and R. S. Weber, Bioresour. Technol., 2012, 109, 154–162 CrossRef CAS PubMed.
- H. J. Huang, X. Z. Yuan, G. M. Zeng, J. Y. Wang, H. Li, C. F. Zhou, X. K. Pei, Q. You and L. Chen, Fuel Process. Technol., 2011, 92, 147–153 CrossRef CAS PubMed.
- D. R. Vardon, B. K. Sharma, J. Scott, G. Yu, Z. C. Wang, L. Schideman, Y. H. Zhang and T. J. Strathmann, Bioresour. Technol., 2011, 102, 8295–8303 CrossRef CAS PubMed.
- X. Z. Yuan, J. Y. Wang, G. M. Zeng, H. J. Huang, X. K. Pei, H. Li, Z. F. Liu and M. H. Cong, Energy, 2011, 36, 6406–6412 CrossRef CAS PubMed.
- U. Jena, K. C. Das and J. R. Kastner, Appl. Energy, 2012, 98, 368–375 CrossRef CAS PubMed.
- U. Jena and K. C. Das, Energy Fuels, 2011, 25, 5472–5482 CrossRef CAS.
- C. Posten and G. Schaub, J. Biotechnol., 2009, 142, 64–69 CrossRef CAS PubMed.
- Q. Q. Guan, C. H. Wei and P. E. Savage, Energy Fuels, 2012, 26, 4575–4582 CrossRef CAS.
- Q. Q. Guan, C. H. Wei and P. E. Savage, Phys. Chem. Chem. Phys., 2012, 14, 3140–3147 RSC.
- Q. Q. Guan, P. E. Savage and C. H. Wei, J. Supercrit. Fluids, 2012, 61, 139–145 CrossRef CAS PubMed.
- P. Azadi and R. Farnood, Int. J. Hydrogen Energy, 2011, 36, 9529–9541 CrossRef CAS PubMed.
- J. A. Onwudili, A. R. L. Langton, A. B. Ross and P. T. Williams, Bioresour. Technol., 2013, 127, 72–80 CrossRef CAS PubMed.
- S. H. Ho, S. W. Huang, C. Y. Chen, T. Hasunuma, A. Kondo and J. S. Chang, Bioresour. Technol., 2013, 135, 191–198 CrossRef CAS PubMed.
- Y. Chisti, Trends Biotechnol., 2008, 26, 126–131 CrossRef CAS PubMed.
- R. Harun and M. K. Danquah, Process Biochem., 2011, 46, 304–309 CrossRef CAS PubMed.
- R. Harun, M. K. Danquah and G. M. Forde, J. Chem. Technol. Biotechnol., 2010, 85, 199–203 CAS.
- M. V. Koopmans, R. H. Wijffels, M. J. Barbosa and M. H. M. Eppink, Bioresour. Technol., 2013, 135, 142–149 CrossRef PubMed.
- S. Arad and O. L. Ontman, Curr. Opin. Biotechnol., 2010, 21, 358–364 CrossRef CAS PubMed.
- D. R. Georgianna and S. P. Mayfield, Nature, 2012, 488, 329–335 CrossRef CAS PubMed.
- V. T. Duong, Y. Li, E. Nowak and P. M. Schenk, Energies, 2012, 5, 1835–1849 CrossRef CAS PubMed.
- X. D. Wu, R. S. Ruan, Z. Y. Du and Y. H. Liu, Energies, 2012, 5, 2667–2682 CrossRef CAS PubMed.
- E. Stephens, I. L. Ross, Z. King, J. H. Mussgnug, O. Kruse, C. Posten, M. Borowitzka and B. Hankamer, Nat. Biotechnol., 2010, 28, 126–128 CrossRef CAS PubMed.
- T. Shirvani, X. Y. Yan, O. R. Inderwildi and P. P. Edwards, Energy Environ. Sci., 2011, 4, 3773–3778 CAS.
- S. H. Al-lwayzy and T. Yusaf, Energies, 2013, 6, 766–783 CrossRef CAS PubMed.
- V. Patil, K. Q. Tran and H. R. Giselrød, Int. J. Mol. Sci., 2008, 9, 1188–1195 CrossRef CAS PubMed.
- E. Suali and R. Sarbatly, Renewable Sustainable Energy Rev., 2012, 16, 4316–4342 CrossRef CAS PubMed.
- L. Brennan and P. Owende, Renewable Sustainable Energy Rev., 2010, 14, 557–577 CrossRef CAS PubMed.
- K. G. Wang, R. C. Brown, S. Homsy, L. Martinez and S. S. Sidhu, Bioresour. Technol., 2013, 127, 494–499 CrossRef CAS PubMed.
- J. X. Zhang, W. T. Chen, P. Zhang, Z. Y. Luo and Y. H. Zhang, Bioresour. Technol., 2013, 133, 389–397 CrossRef CAS PubMed.
- A. Demirbas, Energy Convers. Manage., 2000, 41, 633–646 CrossRef CAS.
- D. R. Vardon, B. K. Sharma, G. V. Blazina, K. Rajagopalan and T. J. Strathmann, Bioresour. Technol., 2012, 109, 178–187 CrossRef CAS PubMed.
- M. Balat, Energy Sources, Part A, 2008, 30, 649–659 CrossRef CAS.
- D. Mohan, C. U. Pittman and P. H. Steele, Energy Fuels, 2006, 20, 848–889 CrossRef CAS.
- H. S. Heo, H. J. Park, Y. K. Park, C. Ryu, D. J. Suh, Y. W. Suh, J. H. Yim and S. S. Kim, Bioresour. Technol., 2010, 101, S91–S96 CrossRef CAS PubMed.
- R. He, X. P. Ye, B. C. English and J. A. Satrio, Bioresour. Technol., 2009, 100, 5305–5311 CrossRef CAS PubMed.
- J. Shen, X. S. Wang and M. G. Perez, Fuel, 2009, 88, 1810–1817 CrossRef CAS PubMed.
- R. Xu, L. Ferrante, C. Briens and F. Berruti, J. Anal. Appl. Pyrolysis, 2009, 86, 58–65 CrossRef CAS PubMed.
- H. J. Park, Y. K. Park and J. S. Kim, Fuel Process. Technol., 2008, 89, 797–802 CrossRef CAS PubMed.
- K. H. Lee, B. S. Kang, Y. K. Park and J. S. Kim, Energy Fuels, 2005, 19, 2179–2184 CrossRef CAS.
- H. J. Park, Y. K. Park, J. I. Dong, J. S. Kim, J. K. Jeon, S. S. Kim, J. Kim, B. Song, J. Park and K. J. Lee, Fuel Process. Technol., 2009, 90, 186–195 CrossRef CAS PubMed.
- G. Duman, C. Okutucu, S. Ucar, R. Stahl and J. Yanik, Bioresour. Technol., 2011, 102, 1869–1878 CrossRef CAS PubMed.
- W. M. Peng, Q. Y. Wu and P. G. Tu, J. Appl. Phycol., 2001, 13, 5–12 CrossRef.
- S. P. Zou, Y. L. Wu, M. D. Yang, C. Li and J. M. Tong, Bioresour. Technol., 2010, 101, 359–365 CrossRef CAS PubMed.
- D. M. Li, L. M. Chen, S. L. Chen, X. W. Zhang, F. J. Chen and N. H. Ye, Fuel, 2012, 96, 185–191 CrossRef CAS PubMed.
- A. Agrawal and S. Chakraborty, Bioresour. Technol., 2013, 128, 72–80 CrossRef CAS PubMed.
- A. Marcilla, A. G. Siurana, C. Gomis, E. Chápuli, M. C. Catalá and F. J. Valdés, Thermochim. Acta, 2009, 484, 41–47 CrossRef CAS PubMed.
- I. V. Babich, M. V. D. Hulst, L. Lefferts, J. A. Moulijn, P. O. Connor and K. Seshan, Biomass Bioenergy, 2011, 35, 3199–3207 CrossRef CAS PubMed.
- S. Grierson, V. Strezov, G. Ellem, R. Mcgregor and J. Herbertson, J. Anal. Appl. Pyrolysis, 2009, 85, 118–123 CrossRef CAS PubMed.
- T. Dickerson and J. Soria, Energies, 2013, 6, 514–538 CrossRef CAS PubMed.
- X. L. Miao and Q. Y. Wu, J. Biotechnol., 2004, 110, 85–93 CrossRef CAS PubMed.
- W. M. Peng, Q. Y. Wu and P. G. Tu, J. Appl. Phycol., 2000, 12, 147–152 CrossRef CAS.
- K. Chaiwong, T. Kiatsiriroat, N. Vorayos and C. Thararax, Biomass Bioenergy, 2013, 56, 600–606 CrossRef CAS PubMed.
- P. Pan, C. W. Hu, W. Y. Yang, Y. S. Li, L. L. Dong, L. F. Zhu, D. M. Tong, R. W. Qing and Y. Fan, Bioresour. Technol., 2010, 101, 4593–4599 CrossRef CAS PubMed.
- A. E. H. Ware, T. Morgan, M. Wilson, M. Crocker, J. Zhang, K. L. Liu, J. Stork and S. Debolt, Renewable Energy, 2013, 60, 625–632 CrossRef PubMed.
- A. Demirbas, Energy Sources, Part A, 2006, 28, 933–940 CrossRef CAS.
- A. Campanella and M. P. Harold, Biomass Bioenergy, 2012, 46, 218–232 CrossRef CAS PubMed.
- Z. Q. Hu, Y. Zheng, F. Yan, B. Xiao and S. M. Liu, Energy, 2013, 52, 119–125 CrossRef CAS PubMed.
- S. T. Gopakumar, S. Adhikari, S. A. Chattanathan and R. B. Gupta, Bioresour. Technol., 2012, 118, 150–157 CrossRef PubMed.
- D. Beneroso, J. M. Bermúdez, A. Arenillas and J. A. Menéndez, Bioresour. Technol., 2013, 144, 240–246 CrossRef CAS PubMed.
- K. M. Holland, US. Pat., 5387321, 1995.
- K. M. Holland, US. Pat., 5330623, 1994.
- A. Marcilla, L. Catalá, J. C. G. Quesada, F. J. Valdés and M. R. Hernández, Renewable Sustainable Energy Rev., 2013, 27, 11–19 CrossRef CAS PubMed.
- X. Y. Li, L. Su, Y. J. Wang, Y. Q. Yu, C. W. Wang, X. L. Li and Z. H. Wang, Front. Environ. Sci. Eng., 2012, 6, 295–303 CrossRef CAS PubMed.
- P. T. Williams and N. Nugranad, Energy, 2000, 25, 493–513 CrossRef CAS.
- Y. Zeng, B. H. Zhao, L. F. Zhu, D. M. Tong and C. W. Hu, RSC Adv., 2013, 3, 10806–10816 RSC.
- T. A. Milne, R. J. Evans and N. Nagle, Biomass, 1990, 21, 219–232 CrossRef CAS.
- H. W. Lee, S. J. Choia, S. H. Park, J. K. Jeon, S. C. Jung, S. H. Joo and Y. K. Park, Energy, 2014, 66, 2–6 CrossRef CAS PubMed.
- Z. Y. Du, X. C. Ma, Y. Li, P. Chen, Y. H. Liu, X. Y. Lin, H. W. Lei and R. S. Ruan, Bioresour. Technol., 2013, 139, 397–401 CrossRef CAS PubMed.
- Z. F. Hu, X. Q. Ma and C. X. Chen, Bioresour. Technol., 2012, 107, 487–493 CrossRef CAS PubMed.
- B. Han, Y. Chen, Y. L. Wu, D. R. Hua, Z. Chen, W. Feng, M. D. Yang and Q. H. Xie, J. Therm. Anal. Calorim., 2013, 115, 227–235 CrossRef PubMed.
- L. M. Zhou, Y. P. Wang, Q. W. Huang and J. Q. Cai, Fuel Process. Technol., 2006, 8, 963–969 CrossRef PubMed.
- A. Aboulkas and E. K. Harfi, J. Therm. Anal. Calorim., 2009, 95, 1007–1013 CrossRef CAS.
- J. Wang, S. Y. Zhang, X. Guo, A. X. Dong, C. Chen, S. W. Xiong, Y. T. Fang and W. D. Yin, Energy Fuels, 2012, 26, 7120–7126 CrossRef CAS.
- V. I. Sharypov, N. G. Beregovtsova, B. N. Kuznetsov, L. Membrado, V. L. Cebolla, N. Marin and J. V. Weber, J. Anal. Appl. Pyrolysis, 2003, 67, 325–340 CrossRef CAS.
- L. Zhang, S. P. Xu, W. Zhao and S. Q. Liu, Fuel, 2007, 86, 353–359 CrossRef CAS PubMed.
- C. X. Chen, X. Q. Ma and Y. He, Bioresour. Technol., 2012, 117, 264–273 CrossRef CAS PubMed.
- Y. T. Tang, X. Q. Ma and Z. Y. Lai, Bioresour. Technol., 2011, 102, 1879–1885 CrossRef CAS PubMed.
- K. Kirtania and S. Bhattacharya, Biomass Bioenergy, 2013, 55, 291–298 CrossRef CAS PubMed.
- W. Li, W. Li and H. Liu, Fuel, 2010, 89, 965–970 CrossRef CAS PubMed.
- S. Yuan, X. L. Chen, W. F. Li, H. F. Liu and F. C. Wang, Bioresour. Technol., 2011, 102, 10124–10130 CrossRef CAS PubMed.
- O. Jorquerra, A. Kiperstok, E. A. Sales, M. Embirucu and M. L. Ghirardi, Bioresour. Technol., 2010, 101, 1406–1413 CrossRef PubMed.
- E. Molina, J. Fernandez, F. G. Acien and Y. Christi, J. Biotechnol., 2001, 92, 113–131 CrossRef CAS.
- C. U. Ugwu, H. Aoyagi and H. Uchiyama, Bioresour. Technol., 2008, 99, 4021–4028 CrossRef CAS PubMed.
- L. Lardon, A. Helias, B. Siave, J. P. Steyer and O. Bernard, Energy Environ. Sci., 2009, 43, 6475–6481 CAS.
- D. C. Elliott, T. R. Hart, A. J. Schmidt, G. G. Neuenschwander, L. J. Rotness, M. V. Olarte, A. H. Zacher, K. O. Albrecht, R. T. Hallen and J. E. Holladay, Algal Res., 2013, 2, 445–454 CrossRef PubMed.
- P. E. Savage, Science, 2012, 338, 1039–1040 CrossRef CAS PubMed.
- H. A. Ruiz, R. M. R. Jasso, B. D. Fernandes, A. A. Vicente and J. A. Teixeira, Renewable Sustainable Energy Rev., 2013, 21, 35–51 CrossRef CAS PubMed.
- P. Biller and A. B. Ross, Biofuels, 2012, 3, 603–623 CrossRef CAS.
- D. G. Archer and P. M. Wang, J. Phys. Chem. Ref. Data, 1990, 19, 371–411 CrossRef CAS PubMed.
- A. V. Bandura and S. N. Lvov, J. Phys. Chem. Ref. Data, 2006, 35, 15–30 CrossRef CAS PubMed.
- M. Pham, L. Schideman, J. Scott, N. Rajagopalan and M. J. Plewa, Environ. Sci. Technol., 2013, 47, 2131–2138 CrossRef CAS PubMed.
- P. Biller, A. B. Ross, S. C. Skill, A. L. Langton, B. Balasundaram, C. Hall, R. Riley and C. A. Llewellyn, Algal Res., 2012, 1, 70–76 CrossRef CAS PubMed.
- S. P. Zou, Y. L. Wu, M. D. Yang, C. Li and J. M. Tong, Energy Environ. Sci., 2010, 3, 1073–1078 CAS.
- J. L. Faeth, P. J. Valdez and P. E. Savage, Energy Fuels, 2013, 27, 1391–1398 CrossRef CAS.
- G. Yu, Y. H. Zhang, L. Schideman, T. L. Funk and Z. C. Wang, Trans. ASABE, 2011, 54, 239–246 CrossRef.
- U. Jena, K. C. Das and J. R. Kastner, Bioresour. Technol., 2011, 102, 6221–6229 CrossRef CAS PubMed.
- P. G. Duan, X. J. Bai, Y. P. Xu, A. Y. Zhang, F. Wang, L. Zhang and J. Miao, Bioresour. Technol., 2013, 136, 626–634 CrossRef CAS PubMed.
- D. Zhou, L. Zhang, S. C. Zhang, H. B. Fu and J. M. Chen, Energy Fuels, 2010, 24, 4054–4061 CrossRef CAS.
- J. X. Guo, Y. B. Zhuang, L. M. Chen, J. H. Liu, D. M. Li and N. H. Ye, Bioresour. Technol., 2012, 120, 19–25 CrossRef CAS PubMed.
- S. P. Zou, Y. L. Wu, M. D. Yang, C. Li and J. M. Tong, Energy Fuels, 2009, 23, 3753–3758 CrossRef CAS.
- C. Yang, L. S. Jia, C. P. Chen, G. F. Liu and W. P. Fang, Bioresour. Technol., 2011, 102, 4580–4584 CrossRef CAS PubMed.
- T. Matsui, A. Nishihara, C. Ueda, M. Ohtsuki, N. Ikenaga and T. Suzuki, Fuel, 1997, 76, 1043–1048 CrossRef CAS.
- U. Jena, N. Vaidyanathan, S. Chinnasamy and K. C. Das, Bioresour. Technol., 2011, 102, 3380–3387 CrossRef CAS PubMed.
- P. J. Valdez, J. G. Dickinson and P. E. Savage, Energy Fuels, 2011, 25, 3235–3243 CrossRef CAS.
- M. F. Demirbas, Appl. Energy, 2011, 88, 3473–3480 CrossRef CAS PubMed.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef CAS PubMed.
- J. Akhtar and N. A. S. Amin, Renewable Sustainable Energy Rev., 2011, 15, 1615–1624 CrossRef CAS PubMed.
- S. Sawayama, S. Inoue, Y. Dote and S. Y. Yokoyama, Energy Convers. Manage., 1995, 36, 729–731 CrossRef CAS.
- C. Jazrawi, P. Biller, A. B. Ross, A. Montoya, T. Maschmeyer and B. S. Haynes, Algal Res., 2013, 2, 268–277 CrossRef PubMed.
- M. Watanabe, Y. Aizawa, T. Iida, T. M. Aida, C. Levy, K. Sue and H. Inomata, Carbohydr. Res., 2005, 340, 1925–1930 CrossRef CAS PubMed.
- B. Y. Yang and R. Montgomery, Carbohydr. Res., 1996, 280, 27–45 CrossRef CAS.
- W. S. L. Mok, M. J. Antal and G. Varhegyi, Ind. Eng. Chem. Res., 1992, 31, 94–100 CrossRef CAS.
- M. Watanabe, T. Iida, Y. Aizawa, H. Ura, H. Inomata and K. Arai, Green Chem., 2003, 5, 539–544 RSC.
- A. Banerjee, R. Sharma, Y. Chisti and U. C. Banerjee, Crit. Rev. Biotechnol., 2002, 22, 245–270 CrossRef CAS PubMed.
- Y. B. Zhuang, J. X. Guo, L. M. Chen, D. M. Li, J. H. Liu and N. H. Ye, Bioresour. Technol., 2012, 116, 133–139 CrossRef CAS PubMed.
- C. B. Xu and T. Etcheverry, Fuel, 2008, 87, 335–345 CrossRef CAS PubMed.
- P. G. Duan, B. B. Jin, Y. P. Xu, Y. Yang, X. J. Bai, F. Wang, L. Zhang and J. Miao, Bioresour. Technol., 2013, 133, 197–205 CrossRef CAS PubMed.
- P. S. Nigam and A. Singh, Prog. Energy Combust. Sci., 2011, 37, 52–68 CrossRef CAS PubMed.
- J. F. Brennecke and C. A. Eckert, AIChE J., 1989, 35, 1409–1427 CrossRef CAS.
- Y. Chen, Y. L. Wu, P. L. Zhang, D. R. Hua, M. D. Yang, C. Li, Z. Chen and J. Liu, Bioresour. Technol., 2012, 124, 190–198 CrossRef CAS PubMed.
- X. Z. Yuan, H. Li, G. M. Zeng, J. Y. Tong and W. Xie, Energy, 2007, 32, 2081–2088 CrossRef CAS PubMed.
- S. Lamanna and R. Cannistraro, Chem. Phys., 1996, 213, 95–110 CrossRef.
- J. H. Lee and N. R. Foster, J. Ind. Eng. Chem., 1999, 5, 116–122 CAS.
- J. Gao, J. Am. Chem. Soc., 1993, 115, 6893–6895 CrossRef CAS.
- R. L. Yang, Y. Chen, Y. L. Wu, D. R. Hua, M. D. Yang, C. Li, Z. Chen and J. Liu, Energy Fuels, 2013, 27, 2619–2627 CrossRef CAS.
- X. K. Pei, X. Z. Yuan, G. M. Zeng, H. J. Huang, J. Y. Wang, H. Li and H. N. Zhu, Fuel Process. Technol., 2012, 93, 35–44 CrossRef CAS PubMed.
- S. Yin, R. Dolan, M. Harris and Z. Tan, Bioresour. Technol., 2010, 101, 3657–3664 CrossRef CAS PubMed.
- A. Kruse, P. Maniam and F. Spieler, Ind. Eng. Chem. Res., 2007, 46, 87–96 CrossRef CAS.
- P. J. L. Williams and L. M. L. Laurens, Energy Environ. Sci., 2010, 3, 554–590 CAS.
- A. Singh, P. S. Nigam and J. D. Murphy, Bioresour. Technol., 2011, 102, 10–16 CrossRef CAS PubMed.
- S. K. Hoekman, A. Broch, C. Robbins, E. Ceniceros and M. Natarajan, Renewable Sustainable Energy Rev., 2012, 16, 143–169 CrossRef CAS PubMed.
- C. Torri, L. G. Alba, C. Samorì, D. Fabbri and D. W. F. Brilman, Energy Fuels, 2012, 26, 658–671 CrossRef CAS.
- Y. N. Liang, Appl. Energy, 2013, 104, 860–868 CrossRef CAS PubMed.
- L. X. Xu, D. W. F. Brilman, J. A. M. Withag, G. Brem and S. Kersten, Bioresour. Technol., 2011, 102, 5113–5122 CrossRef CAS PubMed.
- P. M. Mortensen, J. D. Grunwaldt, P. A. Jensena, K. G. Knudsenc and A. D. Jensen, Appl. Catal., A, 2011, 407, 1–19 CrossRef CAS PubMed.
- J. X. Han, H. Sun, Y. Q. Ding, H. Lou and X. M. Zheng, Green Chem., 2010, 12, 463–467 RSC.
- J. Lu, S. Behtash and A. Heyden, J. Phys. Chem. C, 2012, 116, 14328–14341 CAS.
- D. Kubička and L. Kaluža, Appl. Catal., A, 2010, 372, 199–208 CrossRef PubMed.
- C. Zhao, J. Y. He, A. A. Lemonidou, X. B. Li and J. A. Lercher, J. Catal., 2011, 280, 8–16 CrossRef CAS PubMed.
- P. G. Duan and P. E. Savage, Energy Environ. Sci., 2011, 4, 1447–1456 CAS.
- Z. Li and P. E. Savage, Algal Res., 2013, 2, 154–163 CrossRef PubMed.
- A. Modak, A. Deb, T. Patra, S. Rana, S. Maity and D. Maiti, Chem. Commun., 2012, 48, 4253–4255 RSC.
- P. M. Arvela, I. Kubickova, M. Snåre, K. Eränen and D. Y. Murzin, Energy Fuels, 2007, 21, 30–41 CrossRef.
- F. H. Mahfud, F. Ghijsen and H. J. Heeres, J. Mol. Catal. A: Chem., 2007, 264, 227–236 CrossRef CAS PubMed.
- W. J. Yu, Y. Tang, L. Y. Mo, P. Chen, H. Lou and X. M. Zheng, Bioresour. Technol., 2011, 102, 8241–8246 CrossRef CAS PubMed.
- J. Fu, F. Shi, L. T. J. Thompson, X. Y. Lu and P. E. Savage, ACS Catal., 2011, 1, 227–231 CrossRef CAS.
- J. Fu, X. Lu and P. E. Savage, ChemSusChem, 2011, 4, 481–486 CrossRef CAS PubMed.
- S. Lestari, I. Simakova and A. Tokarev, Catal. Lett., 2008, 122, 247–251 CrossRef CAS.
- M. Snåre, I. Kubičková, P. M. Arvela, K. Eränen and D. Y. Murzin, Ind. Eng. Chem. Res., 2006, 45, 5708–5715 CrossRef.
- S. Lestari, P. M. Arvela, H. Bernas, O. Simakova, R. Sjöholm, J. Beltramini, G. Q. M. Lu, J. Myllyoja, I. Simakova and D. Y. Murzin, Energy Fuels, 2009, 23, 3842–3845 CrossRef CAS.
- J. G. Na, B. E. Yi, J. K. Han, Y. K. Oh, J. H. Park, T. S. Jung, S. S. Han, H. C. Yoon, J. N. Kim, H. Lee and C. H. Ko, Energy, 2012, 47, 25–30 CrossRef CAS PubMed.
- J. G. Na, J. K. Han, Y. K. Oh, J. H. Park, T. S. Jung, S. S. Han, H. C. Yoon, S. H. Chung, J. N. Kim and C. H. Ko, Catal. Today, 2012, 185, 313–317 CrossRef CAS PubMed.
- R. W. Gosselink, D. R. Stellwagen and J. H. Bitter, Angew. Chem., Int. Ed., 2013, 52, 5089–5092 CrossRef CAS PubMed.
- E. S. Jimenez, T. Morgan, J. Lacny, S. Mohapatra and M. Crocker, Fuel, 2013, 103, 1010–1017 CrossRef PubMed.
- C. Torri, D. Fabbri, L. G. Alba and D. W. F. Brilman, J. Anal. Appl. Pyrolysis, 2013, 101, 28–34 CrossRef CAS PubMed.
- B. X. Peng, Y. Yao, C. Zhao and J. A. Lercher, Angew. Chem., Int. Ed., 2012, 51, 2072–2075 CrossRef CAS PubMed.
- B. X. Peng, X. G. Yuan, C. Zhao and J. A. Lercher, J. Am. Chem. Soc., 2012, 134, 9400–9405 CrossRef CAS PubMed.
- B. X. Peng, C. Zhao, S. Kasakov, S. Foraita and J. A. Lercher, Chem.–Eur. J., 2013, 19, 4732–4741 CrossRef CAS PubMed.
- P. G. Duan and P. E. Savage, Appl. Catal., B, 2011, 104, 136–143 CrossRef CAS PubMed.
- P. G. Duan and P. E. Savage, Bioresour. Technol., 2011, 102, 1899–1906 CrossRef CAS PubMed.
- P. G. Duan, X. J. Bai, Y. P. Xu, A. Y. Zhang, F. Wang, L. Zhang and J. Miao, Fuel, 2013, 109, 225–233 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |