Fungi-derived hierarchically porous carbons for high-performance supercapacitors†
Jiacheng Wang* and
Qian Liu*
State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, P. R. China. E-mail: jiacheng.wang@mail.sic.ac.cn; qianliu@sunm.shcnc.ac.cn; Fax: +86-21-52413122; Tel: +86-21-52412714
First published on 9th December 2014
Abstract
Hierarchical porous activated carbons (ACs) were prepared via a chemical activation procedure with sustainable, renewable biomass fungi as carbon precursor and KOH as activating reagent. The as-produced porous ACs present not only a hierarchical porous structure containing macroporous frameworks and microporous textures, but also a high specific surface area of up to 2264 m2 g−1, a large pore volume of up to 1.02 cm3 g−1, and adjustable heteroatom doping (nitrogen: 2.15–4.75 wt%; oxygen: 8.53–14.48 wt%). The microstructural features can be easily controlled by adjusting the mass ratio of KOH/carbon precursor. The porous ACs possess a specific capacitance of up to 158 F g−1 in organic electrolyte, which significantly outperforms the commercially available ACs. The fungi-based ACs electrode also retains 93% of the specific capacitance as the current density increases from 0.1 to 5 A g−1, and has superior cycling performance (92% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).
000 cycles).
Introduction
Supercapacitors, also known as electric double-layer capacitors (EDLC) or ultracapacitors, are a unique type of high-power electrochemical energy storage device that can store energy by forming a double layer of electrolyte ions on the surface of conductive electrodes.1 Recently, supercapacitors have attracted increasing attention due to their reversible charge–discharge, high power density, and long cycle stability. The power delivery performance of supercapacitors makes them perfectly fill the gap between batteries and dielectric capacitors. Thus, supercapacitors are highly attractive in many applications, such as backup energy devices to batteries, customer electronics, hybrid electric vehicles, etc.2As the most key components in supercapacitors, the electrode materials can highly affect the capacitance performance of supercapacitors.3 Among a wide range of the electrode materials studied in supercapacitors, porous carbon materials have attracted much attention because they have many desirable advantages of variable morphologies, low cost, adjustable porosity, lightweight, easy processability, fast adsorption kinetics, high chemical and thermal stability, and controllable heteroatom doping.3a,4 A large specific surface area of porous carbon is very important to good capacitance performance of supercapacitors because energy storage is based on the adsorption of electrolyte ions on the surface area of porous carbon electrode. A traditional porous activated carbon (AC) material with specific surface area of about 1000 m2 g−1 and pore size of 2 to 5 nm has a gravimetric capacitance of 100 to 120 F g−1 in organic electrolytes.5 In order to improve the performance of porous carbon electrodes, much attention was paid to design and synthesize porous carbons with varied pore structure and morphology including various one-dimensional (1D), 2D, and 3D carbonaceous materials. Some nanostructured carbons like carbon nanotubes, carbon onions, etc., have great potentials in supercapacitor applications in case of needing ultrafast rate capability.6 However, the limited specific surface areas of these carbons decrease the energy density of the prepared devices. A large specific surface area in combination with controllable pore structure is high desirable in porous carbons as supercapacitor electrodes. Normally, the optimum pore size leading to the maximum capacitance of porous carbon in ionic liquid electrolyte is roughly equal to the electrolyte ion size (ca. <1 nm) because of effective strengthen of electrolyte–electrode interactions.7
The formation of porous carbon materials with large surface area and well-defined pore size can be realized by the template method, physical activation, and chemical activation.4a,8 The chemical activation using KOH as activating reagent is the most efficient procedure to prepared porous ACs with very high specific surface areas (e.g. 3000 m2 g−1) and well-defined narrow micropore sizes.9 At present, various carbonaceous materials including nanostructured carbons, fossil-based materials, synthetic organic polymers, and low-price natural biomass materials were used as carbon precursors for producing porous ACs with adjustable microstructures.4b By exfoliation of graphite, followed by activation with KOH, Zhu synthesized graphene-based porous carbon with high specific surface area of 3100 m2 g−1, which offered a good capacitance of 167 F g−1 in organic electrolyte.10 It has been reported that the introduction of heteroatoms (e.g. N, O, S, P, etc.), especially nitrogen, into the framework of carbon will improve the capacitance of porous carbon electrodes due to so-called pseudocapacitance while retaining the intrinsic characteristics of porous carbon.11 Therefore, it is of much interest to study the capacitance performance of nitrogen-doped porous carbons with high specific surface areas and well-defined micropore size. The introduction of nitrogen atoms into carbon can be achieved by post-treatment of carbon with nitrogen sources (e.g. ammonia12) and direct carbonization/activation of nitrogen-containing polymers, such as imine-linked polymer,13 polyacrylonitrile,14 polyaniline,15 phenol–formaldehyde,16 and resorcinol–formaldehyde,17 etc.18 However most of these polymers are synthesized in toxic, flammable organic solvents and the procedure is not environmentally friendly and sustainable. Another choice of preparing nitrogen-doped ACs is the use of easily available, low-price, natural biomass precursors as both nitrogen and carbon sources. The chemical activation of hydrothermally carbonized glucose–graphene oxide hybrids resulted in activated carbon xerogels with a cellular morphology which demonstrate high adsorption ability for CO2 and dye.19 This strategy was also utilized to produce natural biomass-derived nitrogen-doped porous ACs with well-developed porosity and high specific capacitance (>140 F g−1) in organic electrolyte.20 Well-known fungi, growing in worldwide abundance as the starting materials in food and pharmaceutical industries, play a key role different from other plants and animals. However their potential applications in material science have attracted little attention so far,21 although they can be supplied in huge amount with sufficient uniformity and consistency.
In this paper, we report the preparation of nitrogen-doped hierarchical porous ACs via a facile chemical activation route, employing natural biomass fungi as carbon precursor and KOH as activating reagent. The as-formed ACs shows large surface areas (2264 m2 g−1), narrow well-defined micropore sizes, and variable nitrogen and oxygen contents, as well as the combination of macroporous frameworks and microporous textures. This unique feature is highly suitable for fast diffusion of organic electrolyte ions into the electroactive surface of micropores. We further demonstrate that the fungi-based porous ACs show high specific capacitance, excellent rate performance and long-term stability in organic electrolyte (1 M tetraethylammonium tetrafluoroborate (TEABF4) in acetonitrile (AN)). The capacitive performance is significantly higher than that reported in commercial ACs,22 and comparable to those for carbide-derived carbons (CDCs) and activated porous graphene.10,23
Experimental
Material preparation
Fresh Agaricus (Fig. S1†) was washed with deionized water to remove the impurities, cut into small pieces, and then dried at 80 °C. The dry samples were pre-carbonized at 500 °C for 2 h in an argon flow and the temperature was ramped from room temperature to 500 °C at 2 °C min−1. The resulting fungi-based char was ground into power for the further chemical activation. Chemical activation was performed at 700 °C with different KOH/char weight ratios. In a typical activation process, the char was thoroughly mixed with KOH pellets using a mortar with a pestle and then the mixture was put into a ceramic crucible. The mixture was heated to 700 °C for 1 h in an argon flow and the temperature was ramped from room temperature to 700 °C at 3 °C min−1. The activated sample was thoroughly washed several times with 10 wt% HCl to remove inorganic impurities and then large amount of distilled water until neutral pH of the filtrate. The wet porous carbons were finally dried at 120 °C for 2 h in air. The resulting fungi-based activated carbons (ACs) were named as AC-x, where x is the weight ratio of KOH/char (x = 1/2, 2 or 4).Material characterization
Nitrogen adsorption–desorption isotherms were collected at −196 °C using a Quantachrome Autosorb 1C apparatus. Prior to the measurement, the samples were degassed in vacuum at 150 °C for 10 h. Specific surface areas were calculated using the Brunauer–Emmett–Teller (BET) equation (p/p0 = 0.05–0.15). The total pore volume was determined at relative pressure p/p0 = 0.98. The pore size distribution was estimated according to the quenched solid density functional theory (QSDFT) equilibrium model for slit pores using the Autosorb 1.56 software from Quantachrome. The micropore volume and surface area were also calculated by the above DFT model.Transmission electron microscopy (TEM) investigations were performed using a 200 kV TEM FEI Tecnai T20 instrument.
Scanning electron microscopy (SEM) coupled with energy dispersive X-ray analysis (EDX) was performed on a ‘DSM-982 Gemini’ using a BSE (backscattered electron) detector from Zeiss.
Elemental analyses were performed using a EURO EA elemental analyzer, fabricated by EURO VECTOR Instruments.
X-ray photoelectron spectra were recorded on a Kratos Axis Ultra DLD spectrometer equipped with a monochromatic Al KR X-ray source (75–150 W) and analyzer pass energy of 160 eV (for survey scans) or 40 eV (for detailed scans).
Electrochemical tests
All the electrochemical measurements were performed on an IviumStat standard-version electrochemical interface and impedance analyzer (Ivium Technologies, NL). The working electrodes were prepared by mixing carbon samples (95%) and polytetrafluoroethylene (PTFF, 5%) binder. The electrodes were measured in a symmetric setup separated by a polypropylene membrane in a Swagelok cell. 1 M tetraethylammonium tetrafluoroborate (TEABF4) in acetonitrile (AN) were used as electrolyte. The cell device was assembled inside an Ar-filled glove box. Electrochemical characterization of the cell devices was performed using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) experiments.Results and discussion
In present study, fungi-based porous activated carbons (ACs) were prepared by pre-carbonization of fresh, abundant Agaricus as a natural precursor, followed by a standard chemical activation procedure with KOH as the activating reagent. The KOH/fungi-char mass ratio has been reported to be a very important parameter affecting the morphology and pore development as well as the elemental compositions of the final ACs.24 Thus the fungi-based char, obtained by pre-carbonization of fresh fungi, was mechanically mixed with KOH pellets at different KOH/char weight ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 4
1 or 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). During the activation process, the carbon combustion proceeds via a stoichoimetric solid–solid/solid–liquid reaction,25 according to the eqn (1).4b
1). During the activation process, the carbon combustion proceeds via a stoichoimetric solid–solid/solid–liquid reaction,25 according to the eqn (1).4b| 6KOH + 2C → 2K + 3H2 + 2K2CO3 | (1) |
The fungi-based char derived from natural Agaricus has high nitrogen content of 5.55 wt% (Table S1†), determined by chemical elemental analysis. The activation procedure performed at 700 °C successfully transferred the uniformly distributed nitrogen atoms of the char into the final ACs. It was found that 4.75, 3.75, and 2.15 wt% of nitrogen existed in AC-1/2, AC-2, and AC-4, respectively. The nitrogen content in the ACs decreased with increasing the KOH amount used. This is because the increase in the KOH amount could enhance the elimination of heteroelement (e.g. nitrogen and oxygen) during the high-temperature treatment. It is noteworthy that all ACs have very high oxygen contents, although the oxygen contents also decreased with the increase in the KOH/char mass ratio. And AC-4 prepared with KOH/char ratio of 4 still has oxygen content of up to 8.53 wt%. The introduction of nitrogen atoms into the carbon framework will improve the carbonaceous electrode performance.20a Thus, the fungi-based porous ACs with different nitrogen contents were further investigated by the combination of the microstructure and supercapacitor performance in detail.
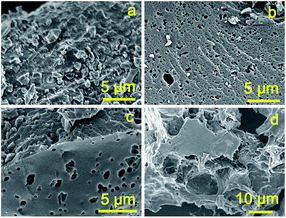
The chemical activation process at high temperature results in not only the evident decrease in the contents of the heteroatoms (nitrogen and hydrogen), but also a significant morphological change in the carbon particles. As shown in Fig. 1a, the fungi-based char obtained by the carbonization of Agaricus at 500 °C shows bulky particles decorated with smaller particles of several micrometers and no any pores can be observed. The activated samples exhibit a quite different morphology from the char (Fig. 1b–d). For example, the resulting AC-1/2, prepared with a KOH/char weight ratio of 1/2, is composed of big particles that have many uniform small cavities (Fig. 1b). AC-2 prepared with an increasing KOH/char weight ratio of 2 (Fig. 1c), has similar morphology to that of AC-1/2 and no significant difference of the morphology between them was observed. With the highest content of KOH used, the as-synthesized AC-4 particles are quite irregular and possess larger distorted cavities (Fig. 1d). The formation of the cavities in the activated samples implies that some carbon was burn off and a dramatic morphological transformation happened during the activation process at high temperature. The yields of the resulting ACs gradually decrease from 34% for AC-1/2 to 25% for AC-2 and 18% for AC-4 (Table 1). The higher amount of KOH used, the lower yield of ACs (Table 1), giving a hint that more carbon was burned off during the activation process. The existence of the macropores in the final carbons offers the fast transportation of the organic electrolyte ions to the smaller pores (e.g. micropores) located at the macropore walls and thus improving the capacitive performance of the carbon-based electrode materials in supercapacitors.
| Sample | Yielda (wt%) | SBET [m2 g−1] | Vp [cm3 g−1] | Smicrob [m2 g−1] | Vmicrob [cm3 g−1] | Dporec [nm] |
|---|---|---|---|---|---|---|
| a Calculated by the weight ratio of AC/char.b Determined by the DFT method.c Maxima of the pore size distribution calculated by the QSDFT. | ||||||
| Char | — | 2 | 0.01 | — | — | — |
| AC-1/2 | 34 | 1479 | 0.71 | 1466 | 0.63 | 0.8 |
| AC-2 | 25 | 1742 | 0.82 | 1581 | 0.80 | 0.8 |
| AC-4 | 18 | 2264 | 1.02 | 1970 | 0.92 | 0.8; 1.4 |
| AC-5 | 5 | 1778 | 1.02 | 1217 | 0.67 | 0.9; 1.8 |
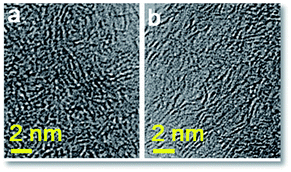
The wide angle X-ray diffraction (XRD) patterns of the fungi-based ACs are shown in Fig. S2.† All ACs prepared with different KOH/char mass ratios demonstrate almost the same curves indicating the similar microstructures in these ACs. These curves have a very broad peak at ∼20° ascribed to the (002) diffraction, showing very low degree of graphitized structure in the ACs. No evident peak at ∼44° corresponding to the (100) diffraction appears, typical of amorphous state in the carbon framework. Also no diffractions due to potassium compounds are observed, confirming the complete removal of potassium impurities by acid washing. The results from the XRD analysis are quite in consistence with the TEM observation showing the disordered graphene layers in these fungi-based ACs (Fig. 2). The formation of the randomly disordered graphene layers allows for high porosity and large surface area in the fungi-based ACs that will be further characterized by nitrogen adsorption measurements at −196 °C.
The mass ratio of the activating reagent and carbon is a very important parameter to develop and finely adjust the pore networks in the ACs. Herein it is found that the pore size and specific surface area of the fungi-based ACs completely depend on the mass ratio of KOH/char. Fig. 3 shows the nitrogen adsorption isotherms and pore size distributions of the fungi-based char and ACs obtained with different mass ratios of KOH/char. The shapes of the isotherms changes as the KOH/char mass ratio increases from 1/2 to 4, indicating the variations in the pore structure of the ACs (Fig. 3a). It is clear that the un-activated char has a very low nitrogen uptake over the whole relative pressure range, typical for non-porous materials. Upon the chemical activation with KOH, all resulting ACs have very high nitrogen uptakes especially in the region of low relative pressure (p/p0 = 0–0.1), and the adsorption isotherms are type I characteristic for microporous materials. The nitrogen uptake evidently increases with increasing the KOH amount, which indicates the formation of increasing porosity. Moreover, the increase of KOH amount used leads to gradual widening of the adsorption at low relative pressure implying broadening of micropore sizes and the formation of larger micropores in the ACs. This micropore widening can also be confirmed by the QSDFT pore size distribution curves shown in Fig. 3b. The AC-1/2 prepared with KOH/char mass ratio of 1/2 demonstrates one single narrow peak centered at ∼0.8 nm. With the moderate amount of KOH used (KOH/char = 2), the single peak of micropore size distribution is still kept, but becomes broader for AC-2. For AC-4 prepared at the highest KOH/char mass ratio of 4, the formation of another wider micropore system (∼1.4 nm) is observed. The pore size distribution curves also imply that the porosity of three ACs prepared with different KOH/char mass ratios are mainly ascribed to micropores (<2 nm). All these results evidently reveal both an increase in specific surface areas and an enlargement of micropore size as the KOH/char mass ratio increases from 1/2 to 4.
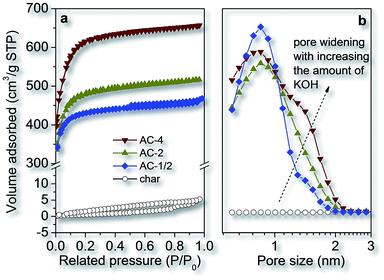 | ||
| Fig. 3 Nitrogen adsorption isotherms (a) and pore size distributions (b) of the fungi-based char and ACs prepared with different KOH/char weight ratios. | ||
Table 1 summarizes the yields and textural properties of the fungi-based char and ACs. The total Brunauer–Emmett–Teller (BET) specific surface area and pore volume of the un-activated char are as low as 2 m2 g−1 and 0.01 cm3 g−1, respectively.26 It can be seen that the specific surface area and pore volume increase evidently with the KOH/char mass ratio from 1479 m2 g−1 (AC-1/2) to 2264 m2 g−1 and from 0.71 cm3 g−1 (AC-1/2) and 1.02 cm3 g−1 (AC-4), respectively. The micropore surface area and volume also increase simultaneously and large proportion of total porosity arises from micropore (Table 1). The increase of the surface area and pore volume as well as the pore widening with increasing the KOH amount used are due to the greater formation of K2CO3 via the reaction between carbon and KOH. Thus more amount of carbon is etched off and then removed by washing with HCl solution. This explanation also match well with the gradual decrease in the carbon yields from 34% for AC-1/2 to 24% for AC-2, 18% for AC-4, and 5% for AC-5, respectively as the amount of KOH used increases (Table 1). The pore properties of surface area and pore volume as well as micropore surface area and volume increase to a maximum for the AC prepared with KOH/char mass ratio = 4, then evidently decreasing for AC prepared with higher mass ratio of KOH/char = 5 used due to the over-activation (Table 1). And the formation of larger micropores (1.8 nm) is also observed for AC-5. The SSA value (2264 m2 g−1) obtained for the fungi-based AC-4 is over 4.5 times higher than that of porous carbons hydrothermally synthesized with the assistance of sacrificial templates,27 and also higher than those of various KOH-activated nanostructured carbons, such as FDU-15 (1410 m2 g−1),28 carbon nanotubes (644 m2 g−1),29 carbon nanofibers (1520 m2 g−1),30 carbide-derived carbons (1650 m2 g−1)31 and carbon aerogels (1468 m2 g−1).32 Moreover, this value is comparable to the specific surface area of activated thermally exfoliated graphene oxide (2400 m2 g−1),10 metal–organic framework-based porous carbon (2222 m2 g−1),33 pig bone-derived carbon (2157 m2 g−1),34 porous ACs derived from organic small molecules,35 and various hydrothermally carbonized renewable organic materials (2000–2300 m2 g−1).36 Recently, the fungi-based ACs with high microporosity are found to be excellent sorbents for CO2 adsorption and separation with superior CO2/N2 selectivity,21 and have great potential as H2 on-board storage media.37
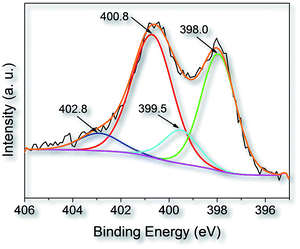
X-ray photoelectron spectroscopy (XPS) is used to further study the nature of nitrogen-containing functional groups on the surface of fungi-based porous carbon particle. Quantitative elemental analysis by XPS shows that the nitrogen content of AC-4 is 2.27 wt%, very close to that obtained by chemical elemental analysis. It presents that the concentration of the surface functional groups in porous carbon is similar to that in the bulk. As shown in Fig. 4, the high-resolution XPS N1s spectrum were deconvolved into four components, which are assigned to pyridinic (N-6, 35.6%) nitrogen at 398.0 eV, pyrrolic/pyridine (N-5, 13.3%) nitrogen at 399.5 eV, quaternary (N-Q, 40%) nitrogen at 400.8 eV, and oxidized nitrogen (N-X, 11.1%) at 402.8 eV, respectively.38 It is worthy to note that the content of N-5 and N-6 that are located on the edge dominates a value of 48.9% in the whole nitrogen functionalities. It has been reported that the most important functional groups affecting energy storage performance are N-5 and N-6 functional groups in the nitrogen-doped porous carbons. Also the N-Q and N-X groups have accelerating effects on the improvement of the capacitance due to the positive charge.38b
The structural characterizations clearly show the unique textural properties of ACs derived from fungi-based char via the chemical activation by KOH. The resulting ACs possess hierarchical porous structure, high specific surface area and pore volume, and varied contents of nitrogen functional groups. Although the previous template methods were adopted to prepare porous carbons, such as nanocasting porous silicas with various carbon precursors in combination of etching off the silica templates using HF solution,39 chlorination of various porous carbides by extracting the silicon element,40 etc., the present one-step activation procedure for producing hierarchical porous carbons are much easier and more environmentally friendly because of use of natural carbonaceous sources that can grow fast and abundantly. Various structural features including specific surface areas, pore volume, pore size, and the content of the heteroatoms of the resultant porous ACs can be rationally tailored by changing the activation parameters (e.g. activation temperature, duration, KOH amount).41
Based on the above results, it can be speculated that the fungi-based ACs have good electrochemical performance because of their high porosity, the interconnectivity of pore structure, and heteroatom-doping. As a promising ammonium salt electrolyte in organic-based supercapacitors,42 TEABF4 in AN was used as electrolyte in present research. Fig. 5 present the CV curves of ACs prepared with different KOH/char ratios at scan rates from 2 to 100 mV s−1. At low scan rate of 2 mV s−1, all CV curves demonstrate quasi-rectangular shape which implies little electrolyte diffusion limitation in characteristic because of high porosity in these fungi-based ACs. Moreover, the clear wide dumps in all the curves are ascribed to the pseudocapacitive contribution derived from the reaction of the electrolyte ions with the surface functional groups.38c,43 These nitrogen/oxygen surface groups can improve the wettability of the carbon surface, and thus significantly enhance the electrochemical active sites on the surface. Also the pseudocapacitive reaction procedure cannot deteriorate the cycle stability of the cell devices. These pseudocapacitive peaks disappeared when the scan rate increased to 100 mV s−1. The quasi-rectangular shape of the CVs still nearly retained, but they clearly shrunk compared to those obtained at 2 mV s−1, implying a significant decrease in the capacitance at higher scan rates. This is very common for porous ACs, and mainly results from the time shortage of ion diffusion and adsorption within the smallest inner pores in the carbon particles.
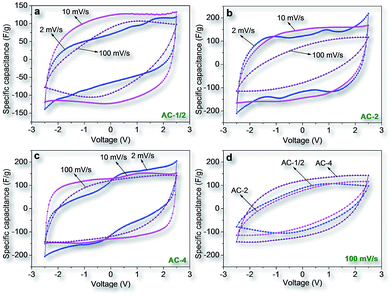 | ||
| Fig. 5 Cyclic voltammograms (CVs) of AC-1/2 (a), AC-2 (b), and AC-4 (c) at different scan rates and the CV comparison for three carbons (AC-1/2, AC-2 and AC-4) at a scan rate of 100 mV s−1. | ||
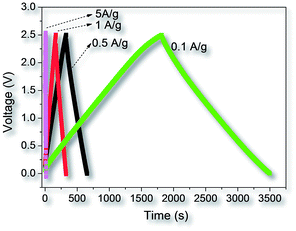
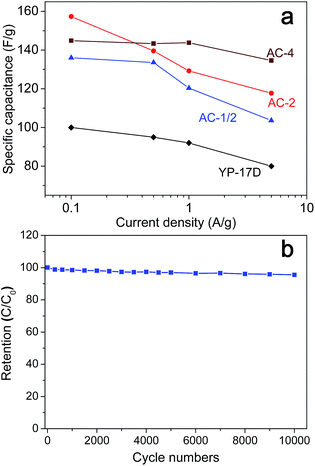
Fig. 6 presents the galvanostatic charge–discharge (GCD) curves of AC-4 at different current density. The GCD curves show a typical, but a little distorted triangular shape, which are due to the pseudocapacitive behaviour of the surface nitrogen and/or oxygen functional groups in the carbon particles. Based on the discharge curve, the gravimetric specific capacitance C (F g−1) can be calculated according to the eqn C = 2I/(m(dV/dt)); where I is the current (A), dV/dt is the slope of the discharge curve (V s−1), and m is the mass (grams) of carbon in each electrode. Fig. 7a shows the relationship of the specific capacitance of AC-1/2, AC-2, and AC-4 with respect to the current density. It can be seen that all capacitance values of these fungi-based ACs significantly exceed the capacitances of conventionally available YP-17D, and are comparable to the capacitances (125–170 F g−1) of the DUT-18 materials,44 and graphene-based porous carbons.10 For low current density (0.1 A g−1), AC-2 demonstrates the highest capacitance value of up to 158 F g−1 among three samples. The specific surface area-normalized capacitances for three samples were in the range of 6.4–9.2 μF cm−2, which are comparable to that of templated mesoporous CDC sample.23b When the testing current density increased from 0.1 to 5 A g−1, AC-1/2 and AC-2 dropped by 23 and 35% in specific capacitances, respectively, while AC-4 only lose about 7% in specific capacitance, implying the high electrochemical stability of sample AC-4. At 5 A g−1, the capacitance value of AC-4 is as high as 135 F g−1, significantly higher than those of AC-1/2 (118 F g−1), AC-2 (103 F g−1), and YP-17D (80 F g−1). The capacitance values of porous carbons are highly related to their microstructures such as pore sizes, surface functional groups, and surface defects. SEM images confirmed that AC-4 prepared at the highest KOH/fungi-char ratio has larger macropores that the ACs prepared at milder conditions (Fig. 1), and nitrogen adsorption measurements confirmed that AC-4 possesses another wider micropore system (∼1.4 nm) compared to AC-1/2 and AC-2 (Fig. 3). Importantly, AC-4 has much higher specific surface area and pore volume that AC-1/2 and AC-2. Hence the unique feature of AC-4 is favourable for the fast diffusion of the organic electrolyte ions to the electroactive sites within the micropores of the carbon particles, which can result in the high specific capacitance value for AC-4 at high current density (5 A g−1). In addition the performance for the supercapacitor with fungi-based ACs as electrode is very stable. Fig. 7b shows the cycling performance of AC-4 at a current density of 5 A g−1. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 galvanostatic charge–discharge cycles, the specific capacitance of AC-4 remains about 92% of the initial capacitance. The cycling stability is compared to nitrogen-doped porous graphene/carbon frameworks in supercapacitor application,45 indicating that biomass-derived AC-4 can be used as highly stable, environmentally friendly electrode material for supercapacitor.
000 galvanostatic charge–discharge cycles, the specific capacitance of AC-4 remains about 92% of the initial capacitance. The cycling stability is compared to nitrogen-doped porous graphene/carbon frameworks in supercapacitor application,45 indicating that biomass-derived AC-4 can be used as highly stable, environmentally friendly electrode material for supercapacitor.
As shown in Fig. 8a, the Nyquist plot of AC-4 was also recorded from 0.01 to 5000 Hz in order to demonstrate the internal resistance of the electrode material. This plot is composed of two parts: a resistance capacitance (RC) semicircle at high frequency and a vertical line at low frequency, presenting the typical feature of porous electrode. The vertical line implies a nearly ideal capacitive behaviour of the supercapacitor. The RC circle in the Nyquist plot is correlative with the diffusion of the ions into the porous electrode particles. A transition between the RC semicircle and the migration of electrolyte was observed at a frequency of about 20 Hz, corresponding to a resistance of 15 ohms. The result implies that the electrode of AC-4 has relatively high internal and charge-transfer resistances than activated porous graphene electrode,10 possibly because the biomass-based porous carbon has high content of oxygen-containing groups. The capacitance from the frequency data as a function of frequency is displayed in Fig. 8b. The capacitance decreases with the increase in frequency.45,46 The specific capacitance retained 70% of the original capacitance at a frequency of 0.1 Hz, while only 5% of the original capacitance was kept with the frequency increased to 10 Hz because of its relatively high resistance.
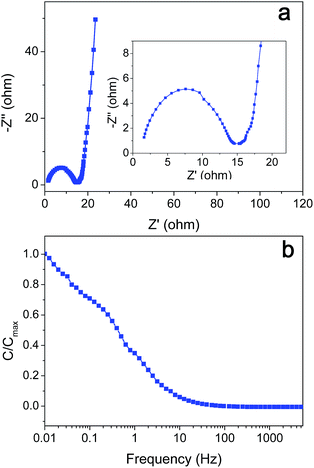 | ||
| Fig. 8 (a) The Nyquist plot at high and low (inset) frequency of impedance spectrum and (b) frequency response of the gravimetric capacitance of AC-4. | ||
Conclusions
In summary, we have described the promising approach to produce nitrogen-doped hierarchical porous carbons by using renewable biomass fungi as carbon source and KOH as activating reagent. The combination of pre-carbonization of clean fungi at 500 °C and chemical activation at 700 °C allowed us to porous ACs showing large specific surface areas (1479–2264 m2 g−1), pore volume (0.71–1.02 cm3 g−1), well-defined micropore sizes, and adjustable nitrogen and oxygen contents (nitrogen: 2.15–4.75 wt%; oxygen: 8.53–14.48 wt%). The microstructural textures of these ACs are easily controlled by the mass ratio of KOH/char. Activation at higher mass ratio of KOH/char (4/1) results in not only the increase of the surface areas, but also the broadening of the micropore size distribution by forming another wider micropore system (∼1.4 nm). As the electrode material for supercapacitors, the fungi-based porous ACs demonstrated specific capacitance of 158 F g−1 in organic electrolytes, which significantly outperform the commercially available ACs, and are comparable to other mesoporous CDCs and activated porous graphene. The porous AC electrode also retained 93% of the specific capacitance as the current density increased from 0.1 to 5 A g−1, and had superior cycling performance (92% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), showing their great potential industrial application in safe, high-energy storage. It is reasonably thought that the fungi-based ACs are very promising potential candidates for high-performance electrode materials in supercapacitors.
000 cycles), showing their great potential industrial application in safe, high-energy storage. It is reasonably thought that the fungi-based ACs are very promising potential candidates for high-performance electrode materials in supercapacitors.
Acknowledgements
This work was supported by Shanghai Institute of Ceramics, the One Hundred Talent Plan of Chinese Academy of Sciences, and National Natural Science Foundation of China (Grant no. 21307145).Notes and references
- (a) M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS; (b) L.-H. Sun, H.-B. Liu, X.-H. Xia and Y.-D. He, J. Inorg. Mater., 2013, 28, 267 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- (a) L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520 RSC; (b) Y.-G. Huang, F.-H. Zheng, M.-D. Ren, Q.-Y. Li and H.-Q. Wang, J. Inorg. Mater., 2013, 28, 1228 CrossRef CAS.
- (a) J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073 CrossRef CAS; (b) J. Wang and S. Kaskel, J. Mater. Chem., 2012, 22, 23710 RSC; (c) M.-G. Deng, R.-Q. Wang and Y.-H. Feng, J. Inorg. Mater., 2014, 29, 245 CAS.
- J. Fernández, T. Morishita, M. Toyoda, M. Inagaki, F. Stoeckli and T. Centeno, J. Power Sources, 2008, 175, 675 CrossRef PubMed.
- (a) Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L.-C. Qin, Phys. Chem. Chem. Phys., 2011, 13, 17615 RSC; (b) E. Frackowiak and F. Beguin, Carbon, 2002, 40, 1775 CrossRef CAS; (c) D. Pech, M. Brunet, H. Durou, P. Huang, V. Mochalin, Y. Gogotsi, P.-L. Taberna and P. Simon, Nat. Nanotechnol., 2010, 5, 651 CrossRef CAS PubMed; (d) Y. Liu, Y.-L. Zhao, Z.-F. Gao, X. Jiang and Y.-P. Zeng, J. Inorg. Mater., 2013, 28, 611 CrossRef CAS.
- C. Largeot, C. Portet, J. Chmiola, P.-L. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730 CrossRef CAS PubMed.
- H. Marsh and F. R. Reinoso, Activated Carbon, Elsevier, Amsterdam, 2006 Search PubMed.
- T. Otowa, R. Tanibata and M. Itoh, Gas Sep. Purif., 1993, 7, 241 CrossRef CAS.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537 CrossRef CAS PubMed.
- (a) D. Hulicova, M. Kodama and H. Hatori, Chem. Mater., 2006, 18, 2318 CrossRef CAS; (b) F. Ma, H. Zhao, L. Sun, Q. Li, L. Huo, T. Xia, S. Gao, G. Pang, Z. Shi and S. Feng, J. Mater. Chem., 2012, 22, 13464 RSC; (c) Y. Zhou, S. L. Candelaria, Q. Liu, Y. Huang, E. Uchaker and G. CaO, J. Mater. Chem. A, 2014, 2, 8472 RSC; (d) Y.-Q. Feng, F.-L. Tang, J.-W. Lang, W.-W. Liu and X.-B. Yan, J. Inorg. Mater., 2013, 28, 677 CrossRef CAS.
- T. Horikawa, N. Sakao, T. Sekida, J. i. Hayashi, D. Do and M. Katoh, Carbon, 2012, 50, 1833 CrossRef CAS PubMed.
- J. Wang, I. Senkovska, M. Oschatz, M. R. Lohe, L. Borchardt, A. Heerwig, Q. Liu and S. Kaskel, ACS Appl. Mater. Interfaces, 2013, 5, 3160 CAS.
- (a) E. Ra, E. Raymundo-Piñero, Y. Lee and F. Béguin, Carbon, 2009, 47, 2984 CrossRef CAS PubMed; (b) K.-P. Wang and H. Teng, J. Electrochem. Soc., 2007, 154, A993 CrossRef CAS PubMed.
- L. Li, E. Liu, J. Li, Y. Yang, H. Shen, Z. Huang, X. Xiang and W. Li, J. Power Sources, 2010, 195, 1516 CrossRef CAS PubMed.
- H. Teng and S.-C. Wang, Carbon, 2000, 38, 817 CrossRef CAS.
- Y. Zhu, H. Hu, W. Li and X. Zhang, Carbon, 2007, 45, 160 CrossRef CAS PubMed.
- A. Castro-Muñiz, F. Suárez-García, A. Martínez-Alonso, J. Tascón and T. Kyotani, ChemSusChem, 2013, 6, 1406 CrossRef PubMed.
- F. J. Martín-Jimeno, F. Suárez-García, J. I. Paredes, A. Martínez-Alonso and J. M. D. Tascón, Carbon, 2015, 81, 137 CrossRef PubMed.
- (a) L. Zhao, L. Z. Fan, M. Q. Zhou, H. Guan, S. Qiao, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 5202 CrossRef CAS PubMed; (b) M.-M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103 RSC.
- J. Wang, A. Heerwig, M. R. Lohe, M. Oschatz, L. Borchardt and S. Kaskel, J. Mater. Chem., 2012, 22, 13911 RSC.
- G. Lota, T. Centeno, E. Frackowiak and F. Stoeckli, Electrochim. Acta, 2008, 53, 2210 CrossRef CAS PubMed.
- (a) M. Oschatz, E. Kockrick, M. Rose, L. Borchardt, N. Klein, I. Senkovska, T. Freudenberg, Y. Korenblit, G. Yushin and S. Kaskel, Carbon, 2010, 48, 3987 CrossRef CAS PubMed; (b) M. Rose, Y. Korenblit, E. Kockrick, L. Borchardt, M. Oschatz, S. Kaskel and G. Yushin, Small, 2011, 7, 1108 CrossRef CAS PubMed.
- M. Sevilla and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 1765 CAS.
- D. Lozano-Castello, J. M. Calo, D. Cazorla-Amoros and A. Linares-Solano, Carbon, 2007, 45, 2529 CrossRef CAS PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- M. M. Titirici, A. Thomas and M. Antonietti, Adv. Funct. Mater., 2007, 17, 1010 CrossRef CAS.
- Y. Lv, F. Zhang, Y. Dou, Y. Zhai, J. Wang, H. Liu, Y. Xia, B. Tu and D. Zhao, J. Mater. Chem., 2012, 22, 93 RSC.
- B. Xu, F. Wu, Y. Su, G. Cao, S. Chen, Z. Zhou and Y. Yang, Electrochim. Acta, 2008, 53, 7730 CrossRef CAS PubMed.
- V. Barranco, M. A. Lillo-Rodenas, A. Linares-Solano, A. Oya, F. Pico, J. Ibanez, F. Agullo-Rueda, J. M. Amarilla and J. M. Rojo, J. Phys. Chem. C, 2010, 114, 10302 CAS.
- C. Portet, M. Angeles Lillo-Rodenas, A. Linares-Solano and Y. Gogotsi, Phys. Chem. Chem. Phys., 2009, 11, 4943 RSC.
- J. Wang, X. Yang, D. Wu, R. Fu, M. S. Dresselhaus and G. Dresselhaus, J. Power Sources, 2008, 185, 589 CrossRef CAS PubMed.
- J. Hu, H. Wang, Q. Gao and H. Guo, Carbon, 2010, 48, 3599 CrossRef CAS PubMed.
- S. Wei, H. Zhang, Y. Huang, W. Wang, Y. Xia and Z. Yu, Energy Environ. Sci., 2011, 4, 736 CAS.
- J. Wang and Q. Liu, Nanoscale, 2014, 6, 4148 RSC.
- M. Sevilla, A. B. Fuertes and R. Mokaya, Energy Environ. Sci., 2011, 4, 1400 CAS.
- J. Wang, I. Senkovska, S. Kaskel and Q. Liu, Carbon, 2014, 75, 372 CrossRef CAS PubMed.
- (a) F. Kapteijn, J. Moulijn, S. Matzner and H.-P. Boehm, Carbon, 1999, 37, 1143 CrossRef CAS; (b) D. Hulicova-Jurcakova, M. Seredych, G. Q. Lu and T. J. Bandosz, Adv. Funct. Mater., 2009, 19, 438 CrossRef CAS; (c) F. Su, C. K. Poh, J. S. Chen, G. Xu, D. Wang, Q. Li, J. Lin and X. W. Lou, Energy Environ. Sci., 2011, 4, 717 RSC.
- A. H. Lu and F. Schüth, Adv. Mater., 2006, 18, 1793 CrossRef CAS.
- (a) R. K. Dash, A. Nikitin and Y. Gogotsi, Microporous Mesoporous Mater., 2004, 72, 203 CrossRef CAS PubMed; (b) V. Presser, M. Heon and Y. Gogotsi, Adv. Funct. Mater., 2011, 21, 810 CrossRef CAS; (c) E. Kockrick, C. Schrage, L. Borchardt, N. Klein, M. Rose, I. Senkovska and S. Kaskel, Carbon, 2010, 48, 1707 CrossRef CAS PubMed; (d) J. Wang, M. Oschatz, T. Biemelt, L. Borchardt, I. Senkovska, M. R. Lohe and S. Kaskel, J. Mater. Chem., 2012, 22, 23893 RSC.
- (a) M. Kubota, A. Hata and H. Matsuda, Carbon, 2009, 47, 2805 CrossRef CAS PubMed; (b) J. Wang, I. Senkovska, M. Oschatz, M. R. Lohe, L. Borchardt, A. Heerwig, Q. Liu and S. Kaskel, J. Mater. Chem. A, 2013, 1, 10951 RSC.
- F. Fusalba, P. Gouérec, D. Villers and D. Bélanger, J. Electrochem. Soc., 2001, 148, A1 CrossRef CAS PubMed.
- B. An, S. Xu, L. Li, J. Tao, F. Huang and X. Geng, J. Mater. Chem. A, 2013, 1, 7222 CAS.
- Y. Korenblit, M. Rose, E. Kockrick, L. Borchardt, A. Kvit, S. Kaskel and G. Yushin, ACS Nano, 2010, 4, 1337 CrossRef CAS PubMed.
- X. Ning, W. Zhong, S. Li, Y. Wang and W. Yang, J. Mater. Chem. A, 2014, 2, 8859 CAS.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Funct. Mater., 2012, 22, 827 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2 and Table S1 mentioned in the text. See DOI: 10.1039/c4ra13358g |
| This journal is © The Royal Society of Chemistry 2015 |