Electrochemical detection of N-homocysteinylated BSA in the fetal bovine serum medium
Ece Eksin and
Arzum Erdem*
Ege University, Faculty of Pharmacy, Analytical Chemistry Department, 35100, Bornova, Izmir, Turkey. E-mail: arzum.erdem@ege.edu.tr; arzume@hotmail.com; Fax: +90-232-388-5258; Tel: +90-232-311-5131
First published on 5th December 2014
Abstract
Homocysteine-thiolactone (HTL) is known as an intramolecular thioester of homocysteine (Hcy). The thioester chemistry of Hcy-thiolactone underlies its ability to form isopeptide bonds with the protein lysine residues, which may impair, or alter the function of protein. HTL modification is a unique post-translational protein modification that is recognized as an emergent biomarker for cardiovascular and neurovascular disease. Electrochemical detection of bovine serum albumin (BSA) before and after N-homocysteinylation was performed using a disposable graphite electrode in combination with a differential pulse voltammetry (DPV) technique. Accordingly, an enhanced detection of BSA was obtained based on the changes at the oxidation signal of BSA after N-homocysteinylation. A lower detection limit was obtained for N-homocysteinylated BSA (N-Hcy-BSA) as 1.09 μg mL−1 in comparison to the one of non-homocysteinylated BSA (i.e., 1.55 μg mL−1). The DL of BSA and N-Hcy-BSA in fetal bovine serum medium was also calculated and found to be 3.29 μg mL−1 and 2.72 μg mL−1, respectively.
Introduction
In healthy humans, some enzymes catalyze the reconversion of homocysteine into either methionine or cysteine. Nevertheless, some conditions, such as a diet particularly rich in methionine; mutations or inactivation of cystathionine β-synthase, methionine synthase, and 5,10-methylene tetrahydrofolate reductase; and nutritional deficiencies of vitamins (folic acid or vitamin B12) induce a dramatic increase of cellular homocysteine concentration, with the resulting increase in the rate of homocysteine-thiolactone (HTL) synthesis and increased accumulation of N-Hcy-protein in humans and mice.1,2HTL is a highly reactive thioester analogue of homocysteine (Hcy) synthesized in vivo via enzymatic aminoacyl-tRNA synthase pathways at a rate proportional to extracellular Hcy concentration.1
HTL could be detrimental because of its ability to acylate proteins under physiological conditions, which would lead to cell damage.3,4 In vitro experimental have shown that HTL readily and selectively reacts with proteins at the ε-amino groups of lysine residues under physiological conditions. This modification may result with a decrease at the biological activity, at solubility, and an increase immunogenicity for affected proteins.5–8 Furthermore, it has been suggested that an increase at HTL synthesis and N-Hcy-protein accumulation is an important risk factor for cardiovascular and neurodegenerative diseases.4–8
Major pathophysiological consequences of protein N-homocysteinylation include increased susceptibility to thrombogenesis (caused by N-Hcy-fibrinogen)9,10 and an autoimmune response elicited by N-Hcy-proteins, associated with stroke and coronary artery disease.6,11,12
Proteins are complex molecules with unique properties, which play many critical roles in the body. Proteins are made up of hundreds or thousands of smaller units called amino acids, which are attached to one another in long chains. The sequence of amino acids determines each protein's unique 3-dimensional structure and its specific function.13 Up to now, considerable research has been focused on studies of protein damage, using methods including HPLC,14 HPLC and an electrical conductivity detector,15 capillary zone electrophoresis,16 differential scanning calorimetry,17 HPLC and a diode array detector,18 fluorescence spectroscopy,19 mass spectrometry20 and radioimmunoassay.21 Many of these methods are laborious, time-consuming, and often requiring the high-cost equipments.
Electrochemical methods have great potential for detecting proteins due to their low cost, simple operation, and fast response.22–27 There are also some reports for electrochemical detection of protein damage.28–30
For the first time, Zhao et al.31 introduced cytochrome c modification with HTL at gold electrode by using electrochemical techniques. Other studies for determination of N-homocysteinylated protein was summarized in Table 1.
| Analyte | Protein | Technique | LOD | Reference |
|---|---|---|---|---|
| Buffer medium | Cytochrome c | DPV, CV | 1 nmol mL−1 | 31 |
| Human plasma | Albumin, fibrinogen | Mass spectrometry | — | 32 |
| Human cell culture | Amyloid β-peptide | CD spectroscopy fluorescence microscopy | — | 33 |
| Human plasma and urine | Plasma proteins | HPLC | — | 34 |
| Rat, mouse, human plasma | Hemoglobin | Mass spectroscopy SDS-PAGE | — | 35 |
| Mice, human tissues | Myoglobin, haemoglobin, cytochrome c, Lys-albumin | ELISA | — | 36 |
| Human sera | Human serum albumin | UV-vis spectrometry | 5.2 mg mL−1 | 37 |
| Animal, human cell culture | Cytochrome c | Mass spectrometry CD spectrometry | — | 38 |
| Fetal bovine serum | Bovine serum albumin | DPV | 2.72 μg mL−1 | Present work |
In comparison to other carbon based electrodes, the pencil graphite electrodes (PGEs) have some advantages such as high electrochemical reactivity, commercial availability, good mechanical rigidity, disposable, low cost, low technology and easy of modification. The PGEs offer the development of single-use sensor platform, which is simpler and faster comparison to other conventional ones requiring polishing or cleaning procedures. In addition, the results obtained by PGEs present a good reproducibility for each individual surface.
We aimed herein to develop a reliable, selective and sensitive single-use protein sensor based on graphite electrode in combination with DPV technique. Using bovine serum albumin as a model protein, the impact of N-homocysteinylation was explored herein on the protein damage. To the best of our knowledge, the electrochemical monitoring of BSA before and after N-homocysteinylation both in the medium of buffer and FBS was reported for the first time in this present study, under the optimum experimental conditions with a good reproducibility and good sensitivity.
Materials and methods
Apparatus
The oxidation signals of HTL and BSA were measured in the same voltammetric scale by using differential pulse voltammetry (DPV) with an AUTOLAB – PGSTAT 302 electrochemical analysis system and GPES 4.9 software package (Eco Chemie, The Netherlands). The raw data were treated using the Savitzky and Golay filter (level 2) of the GPES software, followed by the moving average baseline correction with a peak width of 0.01. The three-electrode system consisted of the pencil graphite electrode (PGE), an Ag/AgCl/3 M KCl reference electrode and a platinum wire as the auxiliary electrode.Reagents
L-Homocysteine thiolactone (HTL), bovine serum albumin (BSA) and fetal bovine serum (FBS) were purchased from Sigma. The stock solutions of HTL and BSA were prepared by dissolving the lyophilisates in fresh ultrapure triple-distilled water and 50 mM phosphate buffer solution (PBS, pH 7.40), respectively. The protein solutions were then stored at −20 °C. The diluted solutions of protein were prepared in 50 mM phosphate buffer solution (PBS, pH 7.40). Other chemicals and reagents were purchased from Sigma-Aldrich and Merck.Procedure
PGE was used for electrochemical detection of HTL and BSA. A Tombow pencil was used as a holder for each new graphite lead. The electrochemical pretreatment of PGEs was performed as in earlier reports39–42 by applying +1.40 V for 30 s in 0.5 M acetate buffer solution (ABS, pH 4.80).The voltammetric measurements were performed before and after following steps (represented also in Scheme 1): (1) the immobilization of BSA onto the surface of PGEs, (2) the immobilization of HTL onto the surface of disposable PGEs, (3) incubation of HTL and BSA in solution phase, then, immobilization of N-homocysteinylated BSA (N-Hcy-BSA) onto the surface of PGEs were performed as mentioned below. All experiments were carried out at room temperature.
Immobilization of BSA onto the surface of PGEs
Pretreated PGEs were immersed into the vials containing 10 μg mL−1 BSA solution for 1 hour. The electrodes were then rinsed with PBS for 10 s to remove nonspecific adsorption from the electrode surface.Immobilization of HTL onto the surface of PGEs
Pretreated PGEs were immersed into the vials containing 0.5 mM HTL solution for 1 hour. The electrodes were then rinsed with PBS for 10 s to remove nonspecific adsorption from the electrode surface.Incubation of BSA and HTL
10 μg mL−1 BSA and 0.5 mM HTL solutions were mixed and incubated at 37 °C by gently mixing (400 rpm) during 3 hours in order to N-homocysteinylation occurrence. PGEs were then immersed into the vials containing N-Hcy-BSA during 1 hour. The electrodes were then rinsed with PBS for 10 s to remove nonspecific adsorption from the electrode surface.The experiments related to BSA N-homocysteinylation were also performed in the fetal bovine serum (FBS) media. Incubation process of BSA and HTL was performed by following the procedure. The solutions of BSA and HTL were prepared by using phosphate buffer (PBS, pH 7.40). Then, 20 μg mL−1 BSA solution was incubated in the presence of HTL in various concentration (0.5, 1, 2 and 4 mM) during 3 hours at 37 °C. After incubation, the samples were diluted in FBS (in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The final BSA concentration was 10 μg mL−1 and final concentrations of HTL were 0.25, 0.5, 1 and 2 μg mL−1. Then, the PGEs were immersed into the samples during 1 hour. These electrodes were then rinsed by PBS for 10 s to remove nonspecific adsorption from the electrode surface.
1). The final BSA concentration was 10 μg mL−1 and final concentrations of HTL were 0.25, 0.5, 1 and 2 μg mL−1. Then, the PGEs were immersed into the samples during 1 hour. These electrodes were then rinsed by PBS for 10 s to remove nonspecific adsorption from the electrode surface.
As the control samples, FBS alone, FBS in the presence of 10 μg mL−1 BSA, and FBS in the presence of HTL at different concentrations were also tested in our study.
Voltammetric transduction
DPV measurements were performed in PBS to measure the oxidation signals of BSA, or HTL by scanning from 0 V to +1.40 V at pulse amplitude of 50 mV and a scan rate of 50 mV s−1. The same voltammetric transduction was performed to monitor the changes in the signal of BSA before and after N-homocysteinylation.Results and discussion
The interaction between homocysteine-thiolactone (HTL) and bovine serum albumin (BSA) was examined according to N-homocysteinylation reaction in earlier reports.3,4 In our study, the voltammetric detection of interaction between HTL and BSA was examined by using DPV technique and single-use graphite electrode. Thus, the detection of protein (BSA)/damaged protein (N-Hcy-BSA) was investigated by monitoring the oxidation signal of BSA before and after its incubation with HTL.N-Hcy-BSA detection in PBS medium
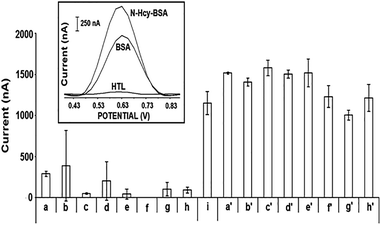
Before interaction process, the oxidation signals of HTL at different concentrations and 10 μg mL−1 BSA were measured respectively at the peak potential of +0.612 V and +0.637 V. After incubation of BSA with HTL during 3 hours, the most increase (37%) at BSA oxidation signal was achieved when BSA incubated with 0.5 mM HTL (Fig. 1). Before incubation with HTL, the average BSA oxidation signal was measured as 1152.25 ± 139.27 nA (RSD% = 12.09%, n = 4). After N-homocysteinylation of BSA with 0.5 mM HTL, the average BSA signal increased to 1578.25 ± 94.20 nA (RSD% = 5.97%, n = 4). Thus, 0.5 mM was chosen as optimum HTL concentration.In order to find optimum incubation time, 10 μg mL−1 BSA and 0.5 mM HTL was incubated at 37 °C during different incubation times; 1, 3, 5 and 8 hours. Before and after incubation, the oxidation signal of BSA/N-Hcy-BSA was measured. Since the highest N-Hcy-BSA signal was obtained when the incubation was performed during 3 hours (not shown), 3 hours incubation time was chosen as optimum incubation time for our further experiments.
The oxidation signal of BSA in its various concentrations from 2.5 μg mL−1 to 15 μg mL−1 was measured before and after incubation of BSA with 0.5 mM HTL during 3 hours at 37 °C in order to determine the optimum concentration of BSA. The highest N-Hcy-BSA signal was achieved when 10 μg mL−1 BSA incubated with 0.5 mM HTL (Fig. 2). Before incubation with HTL, the average oxidation signal of 10 μg mL−1 BSA was measured as 1264.75 ± 192.95 nA (RSD% = 15.26%, n = 8). After N-homocysteinylation of BSA, the average oxidation signal of N-Hcy-BSA was 1570.25 ± 157.27 nA (RSD% = 10.02%, n = 8). Consequently, 10 μg mL−1 was chosen as optimum BSA concentration.
Next, the effect of passive adsorption time of BSA onto the PGE surface was investigated. After the incubation of 10 μg mL−1 BSA with 0.5 mM HTL, the PGE was dipped into the sample that contains N-Hcy-BSA for passive adsorption step during 15 min, 30 min and 60 min. The highest N-Hcy-BSA signal was obtained when 1 hour was applied as passive adsorption time (Fig. 3). Before incubation BSA and HTL, the average oxidation signal was measured as 1286.30 ± 177.55 nA (RSD% = 13.80%, n = 10) for BSA, 60.17 ± 54.91 nA (RSD% = 91.27%, n = 6) for HTL. After N-homocysteinylation, the average oxidation signal of N-Hcy-BSA was obtained as 1677 ± 269.16 nA (RSD% = 16.05%, n = 10).
 | ||
| Fig. 3 Histograms representing the effect of passive adsorption time. The average oxidation signals of (a) 0.5 mM HTL, (b) 10 μg mL−1 BSA, (b′) 10 μg mL−1 BSA after incubation of HTL. | ||
According to Miller and Miller method,43 the detection limit (DL) of BSA was calculated before and after N-homocysteinylation process and it was found respectively as 1.55 μg mL−1 and 1.09 μg mL−1.
N-Hcy-BSA detection in FBS medium
The control experiment was firstly performed in FBS and the average oxidation signal was measured at +0.663 V as 2495.67 ± 299.82 nA (RSD%, 12.01%, n = 9). In the presence of BSA, the signal measured at almost same peak position increased to 2702.80 ± 333.83 nA (RSD%, 12.35%, n = 9). In the presence of HTL in different concentrations; 0.25, 0.5, 1 and 2 mM, the average oxidation signals were measured as 2510 ± 97.58 nA (RSD%, 3.89%, n = 3), 2735.78 ± 274.51 nA (RSD%, 10.03%, n = 3), 2882.33 ± 102.89 nA (RSD%, 3.57%, n = 3), 2763.33 ± 325.98 nA (RSD%, 11.80%, n = 3), respectively. Due to the presence of N-Hcy-BSA production after the interaction of BSA with HTL, there was an increase obtained at the signal in the FBS medium.In the case of interaction of 10 μg mL−1 BSA with 0.25, 0.5, 1 and 2 mM HTL, there was an increase observed respectively about 13.91, 8.86, 22.17, 19.55% increase at the signal (Fig. 4). The most increase ratio% was obtained as 22.17% using 1 mM HTL and it was chosen as the optimum HTL concentration for further experiments.
In order to find optimum incubation time, 10 μg mL−1 BSA and 1 mM HTL was incubated at 37 °C in different incubation times; 1, 3 and 5 hours. Fig. 5 shows the changes ratio% at sensor response regarding to BSA, HTL and after interaction of BSA and HTL in different incubation times. In the case of interaction of BSA and HTL for 1, 3 and 5 h, the increase ratio% was found respectively to be 16.41, 27.25, 15.21%. Since the most increase ratio% was obtained (i.e., 27.25%) for 3 h incubation time, we applied then this incubation time for the further experiments in our study.
Next, we optimized the passive adsorption time of N-Hcy-BSA onto the surface of PGE. After incubation of 10 μg mL−1 BSA and 1 mM HTL during 3 h, the PGEs were immersed into the vials that containing N-Hcy-BSA prepared in FBS during 15 min, 30 min and 1 h. The increase ratio% at the sensor response was found respectively to be 7.14, 10.79, 23.72% (Fig. 6). The most increase ratio% at response was obtained as 23.72% for 1 h passive adsorption time. Accordingly, 1 h passive adsorption time was chosen as a optimum for further experiments.
In order to find optimum BSA concentration, 5, 10, 15, 20 and 25 μg mL−1 BSA solutions were incubated with 1 mM HTL at 37 °C during 3 h. The control experiment was performed firstly and the average oxidation signal was measured as 2522.86 ± 231.59 nA (RSD%, 9.18%, n = 14). After incubation of BSA in various concentrations; 5, 10, 15, 20 and 25 μg mL−1 BSA with HTL, an increase was obtained. The average signals were found to be 2974.50 ± 10.61 nA (RSD%, 0.36%, n = 2), 2860.50 ± 101.12 nA (RSD%, 3.53%, n = 2), 2936.50 ± 27.58 nA (RSD%, 0.94%, n = 2), 2992.33 ± 142.63 nA (RSD%, 4.77%, n = 2), 3103 ± 93.79 nA (RSD%, 3.02%, n = 2), respectively.
In the case of interaction of 10, 15, 20, 25 μg mL−1 BSA with 1 mM HTL, an increase ratio% was found to be 3.92, 6.68, 8.71, 12.73%, respectively. The Fig. 7 represents the calibration plots of BSA before (Fig. 7A), and after N-homocysteinylation (Fig. 7B) in the FBS medium. According to Miller and Miller method,43 the detection limit (DL) of BSA was calculated before and after N-homocysteinylation in the FBS medium, and it was found respectively to be 3.29 μg mL−1 and 2.72 μg mL−1.
Conclusion
The elevated levels of Hcy are an independent risk factor for cardiovascular disease in humans.44 Available data suggest that Hcy can be harmful to human cells because of its metabolic conversion to Hcy thiolactone. This conversion occurs in all human cell types, including endothelial cells. The metabolic conversion of Hcy to HTL, the reactivity of the thiolactone toward proteins by resulting protein damage might be explained some pathologic consequences of elevated Hcy levels, including atherosclerosis.Before and after N-homocysteinylation, the voltammetric detection of BSA was successfully performed in our study by using disposable PGE. The single-use graphite sensor could bring to our study many important advantages, such as, being easy to use resulting with a less time consuming and cheaper detection protocol of HTL, BSA and N-Hcy-BSA. The results introduced herein that homocysteine-thiolactone can bind to proteins and make them more available for oxidation process. Accordingly, a lower DL as 1.09 μg mL−1 was obtained with homocysteinylated BSA than the one (i.e., 1.55 μg mL−1) of non-homocysteinylated BSA. The experiments related to BSA before and after N-homocysteinylation were also performed in the fetal bovine serum (FBS) medium. The DL of N-homocysteinylated BSA was found to be 2.72 μg mL−1, which is lower than the one (i.e., 3.29 μg mL−1) of non-homocysteinylated BSA in the FBS medium.
As a conclusion, our proposed electrochemical detection protocol may possibly be developed further as a practical assay for direct sensitive and selective electrochemical determination of various types damaged proteins.
Acknowledgements
A.E. acknowledges the financial support from the Turkish Scientific and Technological Council (TUBITAK) (Project no. 212T082) and Ege University Science and Technology Research and Application Centre (EBILTEM) (Project no. 2013/BIL/006). E.E. acknowledges a master project scholarship through by TUBITAK (Project no. 212T082).References
- H. Jakubowski, G. H. Boers and K. A. Strauss, FASEB J., 2000, 22, 4071–4076 CrossRef PubMed.
- H. Jakubowski, J. Perla-Kajan, R. H. Finnell, R. M. Cabrera, H. Wang and S. Gupta, FASEB J., 2009, 23, 1721–1727 CrossRef CAS PubMed.
- H. Jakubowski, J. Biol. Chem., 1997, 272, 1935–1942 CAS.
- H. Jakubowski, FASEB J., 1999, 13, 2277–2283 CAS.
- H. Jakubowski, J. Nutr., 2000, 130, 377S–381S CAS.
- H. Jakubowski, Anal. Biochem., 2008, 380, 257–261 CrossRef CAS PubMed.
- A. T. Gates, S. O. Fakayode, M. Lowry, G. M. Ganea, A. Murugeshu, J. W. Robinson, R. M. Strongin and I. M. Warner, Langmuir, 2008, 24, 4107–4113 CrossRef CAS PubMed.
- H. Jakubowski, Clin. Chem. Lab. Med., 2007, 45, 1704–1716 CrossRef CAS PubMed.
- D. L. Sauls, E. Lockhart, M. E. Warren, A. Lenkowski, S. E. Wilhelm and M. Hoffman, Biochemistry, 2006, 45, 2480–2487 CrossRef CAS PubMed.
- A. Undas, J. Brozek, M. Jankowski, Z. Siudak, A. Szczeklik and H. Jakubowski, Arterioscler., Thromb., Vasc. Biol., 2006, 26, 1397–1404 CrossRef CAS PubMed.
- A. Undas, J. Perla, M. Lacinski, W. Trzeciak, R. Kazmierski and H. Jakubowski, Stroke, 2004, 35, 1299–1304 CrossRef CAS PubMed.
- A. Undas, M. Jankowski, M. Twardowska, A. Padjas, H. Jakubowski and A. Szczeklik, Thromb. Haemostasis, 2005, 93, 346–350 CAS.
- M. Krzan, H. Caps and N. Vandewalle, Colloids Surf., A, 2013, 438, 112–118 CrossRef CAS PubMed.
- S. Saeed, D. Gillies, G. Wagner and N. K. Howell, Food Chem. Toxicol., 2006, 44, 1385–1392 CrossRef CAS PubMed.
- K. Hensley, K. S. Williamson, M. L. Maidt, S. P. Gabbita, P. Grammas and R. A. Floyd, J. High Resolut. Chromatogr., 1999, 22, 429–437 CrossRef CAS.
- A. Salvi, P. A. Carrupt, J. P. Tillement and B. Testa, Biochem. Pharmacol., 2001, 61, 1237–1242 CrossRef CAS.
- N. Farkas, J. Belagyi and D. Lőrinczy, Thermochim. Acta, 2003, 404, 141–148 CrossRef CAS.
- Y. Liu, X. Sun, D. Di, J. Quan, J. Zhang and X. Yang, Clin. Chim. Acta, 2011, 412, 2132–2140 CrossRef CAS PubMed.
- K. J. A. Davies and A. L. Goldberg, J. Biol. Chem., 1987, 262, 8227–8234 CAS.
- H. Neumann, J. L. Hazen, J. Weinstein, R. A. Mehl and J. W. Chin, J. Am. Chem. Soc., 2008, 130, 4028–4033 CrossRef CAS PubMed.
- A. C. McGovern, R. Ernill, B. V. Kara, D. B. Kell and R. Goodacre, J. Biotechnol., 1999, 72, 157–167 CrossRef CAS.
- K. Kerman, M. Vestergaard and E. Tamiya, Anal. Chem., 2007, 79, 6881–6885 CrossRef CAS PubMed.
- C. Bian, H. Xiong, X. Zhang, W. Wen and S. Wang, Biosens. Bioelectron., 2011, 28, 216–220 CrossRef CAS PubMed.
- Y. Wang, H. Xiong, X. Zhang, H. Gu and S. Wang, Sens. Actuators, B, 2013, 188, 741–745 CrossRef CAS PubMed.
- M. Chiku, T. A. Ivandini, A. Kamiya, A. Fujishima and Y. Einaga, J. Electroanal. Chem., 2008, 612, 201–207 CrossRef CAS PubMed.
- S. E. Moulton, J. N. Barisci, A. Bath, R. Stella and G. G. Wallace, J. Colloid Interface Sci., 2003, 261, 312–319 CrossRef CAS.
- J. A. Reynaud, B. Malfoy and A. Bere, J. Electroanal. Chem., 1980, 116, 595–606 CrossRef.
- E. Palecek, M. Heyrovsky, B. Janik, D. Kalab and Z. Pechan, Collect. Czech. Chem. Commun., 2009, 74, 1739–1755 CrossRef CAS.
- E. Palecek and V. Ostatna, Analyst, 2009, 134, 2076–2080 RSC.
- V. Ostatna and E. Palecek, Electrochim. Acta, 2008, 53, 4014–4021 CrossRef CAS PubMed.
- J. Zhao, W. Zhu, T. Liu and J. Yang, Anal. Bioanal. Chem., 2010, 397, 695–699 CrossRef CAS PubMed.
- M. Sikora, L. Marczak, J. Kubalska, A. Graban and H. Jakubowski, Amino Acids, 2014, 46, 235–244 CrossRef CAS PubMed.
- S. Khodadadi, G. H. Riazi, S. Ahmadian, E. Hoveizi, O. Karima and H. Aryapour, FEBS Lett., 2012, 586, 127–131 CrossRef CAS PubMed.
- R. Głowacki, E. Bald and H. Jakubowski, Amino Acids, 2011, 41, 187–194 CrossRef PubMed.
- T. Zang, S. Dai, D. Chen, B. W. K. Lee, S. Liu, B. L. Karger and Z. S. Zhou, Anal. Chem., 2009, 81, 9065–9071 CrossRef CAS PubMed.
- J. Perła-Kajan, O. Stanger, M. Luczak, A. Ziołkowska, L. K. Malendowicz, T. Twardowski, S. Lhotak, R. C. Austin and H. Jakubowski, Biomed. Pharmacother., 2008, 62, 473–479 CrossRef PubMed.
- A. T. Gates, S. O. Fakayode, M. Lowry, G. M. Ganea, A. Murugeshu, J. W. Robinson, R. M. Strongin and I. M. Warner, Langmuir, 2008, 24, 4107–4113 CrossRef CAS PubMed.
- J. Perła-Kajan, Ł. Marczak, L. Kajan, P. Skowronek, T. Twardowski and H. Jakubowski, Biochemistry, 2007, 46, 6225–6231 CrossRef PubMed.
- J. Wang and A. N. Kawde, Anal. Chim. Acta, 2001, 431, 219–224 CrossRef CAS.
- A. Erdem, P. Papakonstantinou and H. Murphy, Anal. Chem., 2006, 78, 6656–6659 CrossRef CAS PubMed.
- H. Karadeniz, G. Armagan, A. Erdem, E. Turunc, A. Caliskan, L. Kanit and A. Yalcin, Electroanalysis, 2009, 21, 2468–2476 CAS.
- A. Erdem, H. Karadeniz, G. Mayer, M. Famulok and A. Caliskan, Electroanalysis, 2009, 21, 1278–1284 CrossRef CAS.
- J. N. Miller and J. C. Miller, Statistics And Chemometrics For Analytical Chemistry, Pearson Education, London, 2005 Search PubMed.
- D. W. Jacobsen, Clin. Chem., 1998, 44, 1833–1843 CAS.
| This journal is © The Royal Society of Chemistry 2015 |