Unique self-assembly behavior of amphiphilic block copolymers at liquid/liquid interfaces
Mei Liua,
Yuanyuan Genga,
Qian Wanga,
Yong-Ill Leeb,
Jingcheng Haoa and
Hong-Guo Liu*a
aKey Laboratory for Colloid and Interface Chemistry of Education Ministry, Shandong University, Jinan 250100, P. R. China. E-mail: hgliu@sdu.edu.cn; Tel: +86-531-88362805
bAnastro Laboratory, Department of Chemistry, Changwon National University, Changwon 641-773, Korea
First published on 9th December 2014
Abstract
Diblock copolymers (polystyrene-b-poly(2-vinylpyridine) (PS-b-P2VP)) with different molecular weights self-assembled into various supramolecular microstructures at the polymer chloroform solution/aqueous chloroauric acid interface under different conditions. Generally, multilayered foam films composed of microcapsules with walls decorated with or without round interfacial micelles formed when using a higher concentration of aqueous solution; honeycomb monolayers appeared with decreasing aqueous solution concentration; fish net-like or labyrinthine monolayers were generated with a further decrease in aqueous concentration. The appearance of these microstructures reflects the different adsorption and self-assembling behaviors of PS-b-P2VP including interfacial micellization, encapsulation, and microphase separation under different conditions. In addition, the relative molecular weights of the two blocks and the total molecular weight of the polymers had a large effect on the adsorption and self-assembly of the polymers and on the final microstructure architecture. Furthermore, the factors that affect the adsorption rate and intermolecular interactions of the polymers and, consequentially, the self-assembling behavior and final microstructure are discussed. The catalytic activities of these composite microstructures were evaluated.
1. Introduction
Amphiphilic block copolymers exhibit abundant self-assembly behavior in solutions,1,2 thin films,2,3 and at air/water interfaces.4,5 Micelles, reverse micelles, and vesicles usually form in solutions due to micellization and microphase separation of the polymer molecules. Two-dimensional ordered arrays of micelles or reverse micelles, typically generated in spin-coating, dip-coating, and thin film casting, can interconvert through solvent-annealing processes.6 Due to microphase separation, block copolymers also self-assemble into one-dimensional (1D) microstructures, including parallel and perpendicular cylinders and lamellar structures in thin films. In addition, block copolymers may self-assemble into a pancake-like morphology, strand-like aggregates, ribbons, 2D networks, or cellular patterns at the air/water interface. These self-assembly behaviors make block copolymers a good matrix for incorporation or separation of inorganic nanoparticles used to form functional ordered composite micro- and nanostructures7 and a good template to form ordered structures of inorganic nanoparticles.8Liquid/liquid interfaces that have different microenvironments from solutions and air/water interfaces have been utilized to synthesize inorganic micro- and nanostructures through interfacial reactions9 and to fabricate coordination polymers10 and composites of polymers with various species, including colloidal particles,11 metal ions12 and even graphene oxide13 through adsorption and self-assembly processes. Very recently, we found that some amphiphilic block copolymers adsorbed and interacted with inorganic species including AuCl4−, PtCl62−, and Ag+ ions and formed composite thin films at oil/water interfaces.14–17 We also found that these block copolymers exhibited distinct adsorption and self-assembly behaviors that differed from air/water interface and were greatly affected by the structures of the polymers and the inorganic species in the aqueous phase.
Polystyrene-block-poly(2-vinylpyridine) (PS-b-P2VP) is a typical amphiphilic diblock copolymer with a hydrophobic block PS and a hydrophilic block P2VP. Its micellization behavior in solution has been investigated,18 and micelles and reverse micelles have been used as templates to synthesize hollow SiO2 and TiO2 spheres19 and Au nanoparticles.20 Micelles and reverse micelles of PS-b-P2VP have been used extensively as templates to fabricate two-dimensional (2D) inorganic nanoparticle and nanoring arrays in spin-coating and dip-coating films.21–25 This polymer also self-assembles into ordered cylindrical, 1D periodic lamellar structures and highly ordered circular pores in spin-coating and dip-coating films due to microphase separation of the polymer molecules,26–28 which are often employed to incorporate inorganic nanoparticles in the PS or P2VP microphases used to form ordered structures.29–32 The self-assembly behavior of PS-b-P2VP in pure33,34 and mixed Langmuir monolayers35,36 at the air/water interface has also been widely investigated. However, to the best of our knowledge, no report on the self-assembly of PS-b-P2VP at liquid/liquid interfaces has emerged except for our previous work.14
PS-b-P2VP with 7500 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 molecular weights of the two blocks self-assembled into honeycomb structures at liquid/liquid interfaces formed by polymer chloroform solution with a concentration of 0.2 mg mL−1 and aqueous solutions of HAuCl4 and AgNO3 with concentrations of 1 × 10−3 and 1 × 10−2 mol L−1, respectively.14 This unique structure is different from micro- and nanostructures formed by PS-b-P2VP in solutions, spin-coating or dip-coating films, and Langmuir monolayers and is also different from the structures formed by other amphiphilic block copolymers, such as PS-b-P4VP,15 P2VP-b-PS-b-P2VP,17 P4VP-b-PS-b-P4VP15 and PtBMA-b-P2VP,16 and homopolymer P2VP37–39 at liquid/liquid interfaces. We propose a possible mechanism for the formation of the honeycomb structure that includes adsorption of polymer molecules, formation of building blocks through microphase separation, and further self-assembly of these building blocks.14 Thus, the type of micro- or nanostructure formed depends on the polymer structure and the specific experimental conditions. It is possible that honeycomb is not the sole morphology formed by PS-b-P2VP at the liquid/liquid interface. In order to systematically investigate the self-assembly behavior and regularity of this polymer at the interface and to fabricate functional inorganic/polymer composites, several variations of PS-b-P2VP molecules with different molecular weights were used, and the effects of the concentrations of the inorganic species on the morphologies and structures of the resulting films were analyzed.
500 g mol−1 molecular weights of the two blocks self-assembled into honeycomb structures at liquid/liquid interfaces formed by polymer chloroform solution with a concentration of 0.2 mg mL−1 and aqueous solutions of HAuCl4 and AgNO3 with concentrations of 1 × 10−3 and 1 × 10−2 mol L−1, respectively.14 This unique structure is different from micro- and nanostructures formed by PS-b-P2VP in solutions, spin-coating or dip-coating films, and Langmuir monolayers and is also different from the structures formed by other amphiphilic block copolymers, such as PS-b-P4VP,15 P2VP-b-PS-b-P2VP,17 P4VP-b-PS-b-P4VP15 and PtBMA-b-P2VP,16 and homopolymer P2VP37–39 at liquid/liquid interfaces. We propose a possible mechanism for the formation of the honeycomb structure that includes adsorption of polymer molecules, formation of building blocks through microphase separation, and further self-assembly of these building blocks.14 Thus, the type of micro- or nanostructure formed depends on the polymer structure and the specific experimental conditions. It is possible that honeycomb is not the sole morphology formed by PS-b-P2VP at the liquid/liquid interface. In order to systematically investigate the self-assembly behavior and regularity of this polymer at the interface and to fabricate functional inorganic/polymer composites, several variations of PS-b-P2VP molecules with different molecular weights were used, and the effects of the concentrations of the inorganic species on the morphologies and structures of the resulting films were analyzed.
2. Experimental section
2.1. Chemicals
PS-b-P2VP with two blocks with Mn values of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/12
000/12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (Mw/Mn = 1.06, denoted as P2), 110
500 (Mw/Mn = 1.06, denoted as P2), 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/12
000/12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (Mw/Mn = 1.09, P3), 440
500 (Mw/Mn = 1.09, P3), 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/20
000/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Mw/Mn = 1.2, P4), and 110
000 (Mw/Mn = 1.2, P4), and 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/52
000/52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (Mw/Mn = 1.10, P5) were purchased from Polymer Source (Canada) and were used as received. For comparison, the PS-b-P2VP with Mn values of 7500/12
000 g mol−1 (Mw/Mn = 1.10, P5) were purchased from Polymer Source (Canada) and were used as received. For comparison, the PS-b-P2VP with Mn values of 7500/12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (Mw/Mn = 1.06) that used in our previous work14 was denoted as P1. HAuCl4·3H2O (99.9+%) was purchased from Aldrich. 4-Nitroaniline (≥99.5%) was supplied by Tianjin Kemiou Chem. Reagent Co., Ltd. and KBH4 (≥97.0%) was obtained from Shanghai Zhanyun Chem. Co. Ltd. Chloroform containing 0.3–1.0% ethanol as a stabilizer was obtained from Tianjin Guangcheng Chem. Co. The water used was highly purified using a UP water purification system (UPHW-IV-90T, Chengdu China) with a resistivity ≥18.0 MΩ cm.
500 (Mw/Mn = 1.06) that used in our previous work14 was denoted as P1. HAuCl4·3H2O (99.9+%) was purchased from Aldrich. 4-Nitroaniline (≥99.5%) was supplied by Tianjin Kemiou Chem. Reagent Co., Ltd. and KBH4 (≥97.0%) was obtained from Shanghai Zhanyun Chem. Co. Ltd. Chloroform containing 0.3–1.0% ethanol as a stabilizer was obtained from Tianjin Guangcheng Chem. Co. The water used was highly purified using a UP water purification system (UPHW-IV-90T, Chengdu China) with a resistivity ≥18.0 MΩ cm.
2.2. Generation of micro- and nanostructures at the liquid/liquid interface
Chloroform solutions of the polymers at a concentration of 0.20 mg mL−1 and an aqueous solution of HAuCl4 at a concentration of 1.0 × 10−3 mol L−1 were prepared using weighting methods. Aqueous solutions of HAuCl4 at concentrations of 2.0 × 10−4 and 5.0 × 10−5 mol L−1 were prepared by serial dilutions of the 1.0 × 10−3 mol L−1 stock solution. Then, 5 mL of chloroform solution was poured into a beaker. Next, 5 mL of aqueous solution at a certain concentration was carefully added to the surface of the chloroform solution, resulting in the formation of a clear liquid/liquid interface. The beaker was placed in a sealed container in a dark place. The adsorption and self-assembly process resulted in formation of a thin layer at the interface. After 24 hours, the thin layer was deposited onto solid substrates for further characterization.For deposition of the thin layer, the aqueous phase was carefully removed using a dropping pipette, leaving the formed thin film floating on the oil phase surface. The thin film was deposited onto carbon-coated copper grids, mica sheets, and quartz plates by immersing the solid substrates under the film and slowly lifting them. In order to eliminate the influence of residual aqueous solution on the structure and composition of the film, pure water was added to dilute the aqueous phase. This process was repeated as necessary.
2.3. Characterization
The morphology and structure of thin layers deposited on solid substrates were characterized using transmission electron microscopy (TEM, JEOL-2010) with an accelerating voltage of 200 kV and atomic force microscopy (AFM, Nanoscope IIIa, Digital Instruments Inc.). The electron diffraction (ED) pattern was obtained using HRTEM. Element analysis was carried out using an energy-dispersive spectroscope (EDS; Oxford INCAx-sight) attached to the HRTEM. The compositions of thin layers were probed by using X-ray photoelectron spectroscopy (XPS, ESCALAB MKII) with a Mg Kα exciting source at a pressure of 1.0 × 10−6 Pa and a resolution of 1.00 eV.2.4. Catalytic reaction
Catalytic activities of these thin composite films were evaluated using the reduction of 4-nitroaniline (4-NA) in aqueous solutions. 0.5 mL of aqueous solution of 4-NA with a concentration of 1 × 10−4 mol L−1 was poured into a 1 cm quartz cuvette, then 1.0 mL of aqueous solution of KBH4 with a concentration of 2 × 10−2 mol L−1 was added. The final concentrations of 4-NA and KBH4 in the mixture were 3.33 × 10−5 and 1.33 × 10−2 mol L−1, respectively. The thin films deposited on quartz slides were immersed in the reaction system to catalyze the reduction of 4-NA. The process of reaction was monitored by using UV-vis spectroscopy (Shimadzu, UV2450). The reaction temperature was controlled to be 25 °C through a thermostat.3. Results and discussion
3.1. Morphology and structure
P1 self-assembles into a 2D honeycomb structure at the liquid/liquid interface when using an aqueous solution of HAuCl4 at a concentration of 1.0 × 10−3 mol L−1, as described in our previous work.14 However, P2 forms foam films under the same conditions, as illustrated in Fig. 1. As seen in Fig. 1(a) and (b), two kinds of foam structures were generated at the interface: one is composed of microcapsules with smooth walls, and another is made up of microcapsules with walls decorated with round aggregates. Fig. 1(c) is a high-magnification TEM image of this kind of capsule. The sizes of the microcapsules ranged from several hundreds of nanometers to micrometers, and the size of the round aggregates was about 15 nm. These results clearly reflect the great influence of molecular structure on the self-assembling behaviors of P1 and P2 and the formed microstructures.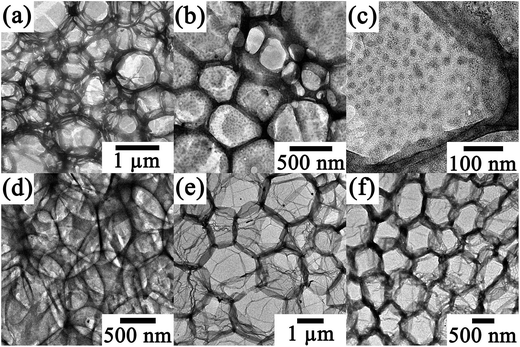 | ||
| Fig. 1 TEM micrographs of composite thin films of P2/Au. The concentration of P2 was 0.2 mg mL−1, and the concentrations of HAuCl4 were 1 × 10−3 (a–c), 2 × 10−4 (d and e), and 5 × 10−5 mol L−1 (f). | ||
Two kinds of morphologies, i.e., a foam structure composed of microcapsules and a honeycomb structure, appeared simultaneously at the interface when the concentration of HAuCl4 aqueous solution was decreased to 2 × 10−4 mol L−1, as exhibited in Fig. 1(d) and (e). When the concentration of the HAuCl4 aqueous solution was further decreased to 5 × 10−5 mol L−1, only a honeycomb structure was observed (Fig. 1(f)). This result suggests that the concentrations of the inorganic species also have a large effect on the self-assembling behavior and the final formed microstructures. The honeycomb structure formed by P2 was similar to that formed by P1,14 composed of polygons with spindle- or shuttle-like sides.
Fig. 2 exhibits TEM micrographs of composite thin films of P3 formed with different HAuCl4 concentrations. Similar to P2, P3 forms a foam structure at higher HAuCl4 concentrations. As seen in Fig. 2(a) and (b), the upper shells of some microcapsules were uncovered, possibly arising from stress elimination due to removal of aqueous phase during the deposition process. All of the walls of the microcapsules were decorated with smaller round aggregates, as shown in Fig. 2(c), which differed slightly from the microstructures formed by P2 under the same conditions (Fig. 1(a) and (b)). Lower HAuCl4 concentrations resulted in a honeycomb structure composed of polygons (Fig. 2(d)), indicating that the polymer molecules adopted another self-assembly pathway under these conditions. With a further decrease in the HAuCl4 concentration, a labyrinthine structure appeared at the interface (Fig. 2(e)). This structure was composed of interconnected lines with lengths of several micrometers that were approximately parallel in some local regions. The high-magnification image shown in Fig. 2(f) shows that the width of the lines was approximately 25 nm, and inorganic nanoclusters formed along both sides of the lines to form 1D arrays. These nanoclusters are made up of Au(0) from reduction of AuCl4− ions by ethanol in the chloroform solution used during the adsorption and assembly process, as observed in our previous work.14–17 These results further reflect the influence of the concentration of AuCl4− ions on the adsorption and self-assembling behavior of polymer molecules at the liquid/liquid interface.
 | ||
| Fig. 2 TEM micrographs of composite thin films of P3/Au. The concentration of P3 was 0.2 mg mL−1, and the concentrations of HAuCl4 were 1 × 10−3 (a–c), 2 × 10−4 (d), and 5 × 10−5 mol L−1 (e and f). | ||
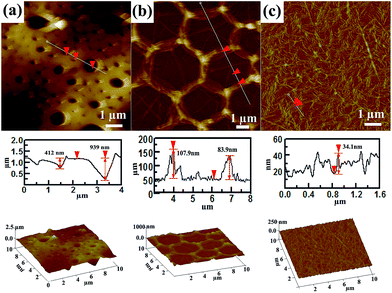
In order to further analyze the structure of the formed thin films of P3/Au under different conditions, these films were investigated using AFM, as shown in Fig. 3. A large number of holes were observed in the AFM image of P3/Au formed at higher HAuCl4 concentration with depths of several hundreds of nanometers to micrometers, similar in size to the microcapsules shown in Fig. 2(a) and (b). This result is consistent with the TEM observations. The holes are presumably formed when the upper shells of microcapsules are removed during the deposition process.
Fig. 3(b) illustrates a typical honeycomb structure that consists of hexagons with sizes of 2 to 3 μm. The sides were spindle-like, with a height of approximately 100 nm. The bottom face was not smooth, with some observable wrinkles. The three-dimensional image shows that the honeycomb holes have six sides. The characteristics of the honeycomb structure obtained from the AFM images were consistent with those from the TEM observations. In addition, the vertexes were higher than the sides.
Fig. 3(c) shows AFM images of the composite nanostructure formed at the interface when the HAuCl4 concentration was further decreased. Roughly parallel nanowires with heights of 30–40 nm appeared, which is in agreement with the TEM observations. Both the AFM and TEM observations confirm that different microstructures formed under the various experimental conditions.
Different microstructures were fabricated under different conditions for P5, as shown in Fig. 4. A foam film composed of microcapsules with smaller round aggregates incorporated into their walls formed with high-concentration HAuCl4 aqueous solution. A plate film decorated with round aggregates formed simultaneously under these conditions. With a decrease in the HAuCl4 concentration to 2 × 10−4 mol L−1, in addition to the foam structure, a honeycomb structure also appeared. A fish net-like structure appeared with a further decrease in the HAuCl4 concentration, as seen from Fig. 4(g–i). Fig. 4(g) gives a panoramic view of this structure, which shows that this fishing net-like pattern was present across the entire film. This film is a monolayer, although two or more layers appeared in some places, which could be attributed to reversion of the film during the deposition process. Fig. 4(h) is an enlarged image that shows some interconnected nanowires with lengths of several hundreds of nanometers that make up the net structure. The high-magnification image in Fig. 4(i) shows that the nanowires had a mean diameter of 25 nm, with nanoclusters densely arranged along the sides to form 1D arrays, and numerous nanoclusters with the size less than 2 nm were dispersed in the mesh. Fig. 4(j) represents the ED pattern of the net structure. Only one diffraction ring can be distinguished, indicating the small size of the formed Au nanoclusters. EDS spectra taken from different regions of the net structure are shown in Fig. 4(k) and (l). Peaks corresponding to gold element appear in these spectra, and the relative contents of Au are close to each other, indicating homogeneous distribution of gold element in the film. Similar to P2 and P3, these results clearly reveal the significant effect of the experimental conditions on the self-assembling behavior of P5 and the final microstructures.
Fig. 1, 2, and 4 show that these polymers self-assembled into different microstructures under the same conditions, implying that the molecular structures of the polymers also have great influence on their self-assembly behaviors. The influences of the experimental conditions and the polymer structures will be discussed below. The relations between molecular structures, concentrations and the formed microstructures were summarized in Table 1.
| Polymer | Mn(PS)–Mn(P2VP) | 1 × 10−3 mol L−1 | 2 × 10−4 mol L−1 | 5 × 10−5 mol L−1 |
|---|---|---|---|---|
| P1 | 7500–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Honeycomb-like | — | — |
| P2 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–12 000–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Foam | Foam + honeycomb-like | Honeycomb-like |
| P3 | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–12 000–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Foam | Honeycomb-like | Labyrinth-like |
| P4 | 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20 000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | — | — |
| P5 | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–52 000–52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Foam + plate film | Foam + honeycomb-like | Fish net-like |
3.2. Composition analysis
The composition of the formed microstructure was checked further using XPS. Fig. 5 represents a typical XPS spectrum of the sample formed by P3 with 5 × 10−5 mol L−1 HAuCl4 solution. The spectrum was decomposed into two pairs of peaks, located at 85.1/88.4 and 87.3/91.7 eV, which were assigned to 4f7/2/4f5/2 of Au(0) in Au nanoclusters and Au(III), respectively.40,41 The generation of Au nanoclusters was ascribed to reduction of AuCl4− ions during the assembly process. This result also suggested that AuCl4− ions were combined successfully in the sample. | ||
| Fig. 5 XPS spectrum of the P3/Au composite structure formed at the liquid/liquid interface at concentrations of 0.2 mg mL−1 P3 and 5 × 10−5 mol L−1 HAuCl4. | ||
3.3. Formation mechanisms of the structures
Three main types of microstructures formed at the liquid/liquid interface through adsorption and self-assembly of the polymers: foam films composed of microcapsules with or without smaller round aggregates on their walls, a honeycomb structure, or a labyrinthine or fish net-like structure formed by nanowires. In general, the formation of these microstructures is attributed to adsorption of the polymer molecules, interaction between the protonated pyridine groups and AuCl4− ions, and self-assembly of the formed composite molecules. In order to reduce interfacial tension, amphiphilic molecules are adsorbed at the liquid/liquid interface, as evidenced by dynamic interfacial tension measurements in a previous report.17 The pH values of the aqueous solutions used here were calculated to be between 3 and 4.5 based on the concentrations of HAuCl4. The pKa value of the conjugate acid of pyridine is 5.3. Thus, the pyridine groups in the adsorbed polymer molecules would be protonated and combine with AuCl4− ions from the aqueous phase to form composite molecules that further self-assemble into microstructures through different approaches. Protonation of the pyridine groups and combination with AuCl4− ions were confirmed through FTIR spectroscopy as in our previous reports.15,37Specifically, the three types of microstructures have their respective formation processes. The formation process for foam films from the block copolymers is similar to that of the corresponding homopolymer P2VP, as described in our previous reports.37–39 Briefly, the composite molecules initially assemble into a thin layer at the interface. Both sides of the thin layer are constituted by P2VP and PS blocks, facing the aqueous and organic phases, respectively. The formation of the thin layer can be considered as a result of microphase separation of the block copolymer at the interface. Due to the stronger interaction between P2VP blocks and the aqueous phase, the thin layer tends to enter the aqueous phase. Thus, the polymer self-assembles into a microcapsule with the outer face composed of hydrophilic P2VP. After formation of the microcapsule, free space allows the generation of another microcapsule through the adsorption and self-assembly process. More microcapsules appear at the interface and accumulate to form a foam film. The smaller round aggregates are interfacial micelles formed by PS-b-P2VP molecules due to the stronger interaction between the PS blocks.
We have previously discussed the formation process of the honeycomb structure.14 Briefly, the composite molecules initially assemble into 1D building blocks at the interface due to microphase separation of the block copolymers. The P2VP blocks intertwine to form a thin layer spread out at the interface due to their hydrophilicity, while the PS blocks also intertwine to form a thin layer beneath the interface due to their hydrophobicity. The thin layer composed of P2VP blocks tends to enter the aqueous phase due to its relative stronger hydrophilicity and interaction with AuCl4− ions, while the thin layer formed by PS blocks exerts a reacting force. Thus, part of the P2VP thin layer is pulled down by this reacting force to form a vertical wall-like structure, and the remainder shrinks at the interface. It can be reasonably deduced that there are some shallow water pools between the walls of these building blocks because P2VP is somewhat hydrophilic. The building blocks further self-assemble into a honeycomb structure around these water pools, and the walls interconnect to form the sides of polygons. The PS thin layers interconnect to form the bottom face.
Apparently, formation of the labyrinthine or fish net-like structure is also the result of microphase separation and self-assembly of composite polymer molecules at the interface. Similar to formation of the honeycomb structure, composite molecules initially organize into 1D building blocks, which then self-assemble into the microstructures. Compared with the honeycomb structure, the walls of the fish net-like structure are thinner and shorter, and the size of the mesh is smaller. In the high-magnification TEM images, numerous nanoclusters disperse into the bottom of the mesh, indicating that the mesh has a bottom face composed of PS blocks, similar to the honeycomb structure. The nanoclusters generated in the bottom of the mesh are attributed to an interaction between HAuCl4, Au nanoclusters and benzene rings because benzene rings have abundant electrons. It has been reported that benzene rings interact strongly with gold colloids,42 and the benzene rings in PS have been utilized as a stabilizer to enclose noble metal clusters.43
3.4. Effects of HAuCl4 concentration
It is very clear that the concentration of HAuCl4 in aqueous solution has a large influence on the microstructure formed at the liquid/liquid interface. With a decreasing concentration of AuCl4−, the pH of the solution increases. Because the adsorption of polymer molecules is driven mainly by protonation of the pyridine groups and the subsequent electrostatic attraction between the protonated groups and complex anions, a decrease in the concentration is unfavorable for adsorption of polymer molecules. Thus, the change in concentration affects the adsorption rate, the interface concentration, and the organization behavior of polymer molecules and, as a consequence, affects the formed microstructure.With higher concentrations of aqueous HAuCl4, polymer molecules were rapidly adsorbed at the interface, the interface concentration of polymer rapidly increased, and the interface became crowded, resulting in the formation of a condensed thin layer composed of two sublayers: an upper layer of P2VP blocks facing the aqueous phase and a lower layer of PS blocks squeezed into the organic phase. In some cases, due to the stronger interaction between PS blocks, interfacial micelles formed among these layers. A stronger interaction between the upper sublayer and the aqueous phase induced further self-assembly of the thin layer, leading to the formation of microcapsules.
The adsorption rate of polymer molecules at the interface slowed with decreasing HAuCl4 concentration. As a consequence, the interface concentration of polymer molecules was lower. Thus, there is sufficient free space for organization of composite molecules. A large number of 1D building blocks were formed in this case. The walls of the building blocks were formed by the P2VP blocks that constitute the sides of the polygons in the honeycomb structure, and the sublayers formed by PS blocks floating near the interface constitute the bottom face of the honeycomb.
With a further decrease in the HAuCl4 concentration, the adsorption rate of polymer molecules slowed significantly. As a result, only a small amount of polymer molecules were adsorbed at the interface. They organized themselves to form nanowires through microphase separation. These nanowires became interconnected, leading to the formation of labyrinthine or fish net-like structures.
It should be noted that, in some cases, two or more microstructures coexist in the same film. The formation of different microstructures under certain conditions is ascribed to the slightly different local microenvironments at the interface. Precise control of the conditions is needed for formation of a single microstructure.
3.5. Effects of polymer molecular structure
It is apparent that the molecular structure of the polymers also has an important effect on the formed microstructure. For example, P1 formed a honeycomb structure with an aqueous HAuCl4 concentration of 1 × 10−3 mol L−1, while P2, P3, and P5 formed a foam structure under the same conditions.The effective adsorption of polymer molecules at the liquid/liquid interface is an essential prerequisite for microstructure fabrication. One of the most important parameters for adsorption is the amphiphilicity of the polymer molecule. Interfacial layer formation for P4 was not observed even when using a high concentration of HAuCl4 because the PS block in this molecule is too long, and the molecule is hydrophobic.
The formula weights of the P2VP blocks in P1, P2, and P3 were equivalent, while the formula weights of the PS blocks in the copolymers were successively increased. Therefore, the P2VP blocks had the same lengths, but the lengths of the PS blocks were different. The polymer molecular structure significantly affects adsorption, interaction, and self-assembly of the molecules. P1 forms a honeycomb structure when using a 1 × 10−3 mol L−1 aqueous solution of HAuCl4,14 while P2 and P3 form foam films, as shown in Fig. 1 and 2. The adsorption rate of P1 at the interface should be higher than that of P2 or P3 because the PS block of P1 is shorter. However, because of the shorter PS chain length, the interaction between adsorbed P1 molecules is weaker than that of P2 or P3. Thus, a delicate balance between adsorption rate and intermolecular interaction determines the self-assembly behavior of the molecules and the final microstructure. A stronger interaction between the longer PS chains of P2 or P3 molecules allows the adsorbed molecules to form a tight film, which further self-assembles into a microcapsule. P1 molecules at the interface form 1D structures through a microphase separation process due to the weaker interaction between PS chains, and these 1D building blocks organize into a honeycomb structure.
This can be also interpreted based on the strength of segregation of the block copolymers at the interfaces. The segregation strength of block copolymers, χN, represents their microphase separation ability, where χ is the segment–segment interaction parameter and N is the degree of polymerization. Generally, χN increases with increasing N. It is apparent that the segregation strength of P2 and P3 is greater than that of P1. So P2 and P3 formed a thin layer at the interface that self-assembled into microcapsule at last. The thin layer composed of two sublayers that consisted of P2VP and PS blocks, respectively. The formation of this double-faced thin layer can be considered as stronger microphase segregation. The segregation strength of P1 is weaker than that of P2 or P3, so 1D building blocks were formed, which assembled into honeycomb structure further.
With a decreased HAuCl4 concentration of 2 × 10−4 mol L−1, P2 forms foam and honeycomb structures, while P3 only forms the honeycomb structure. Although the interaction between adsorbed P2 molecules is weaker than that between P3 molecules because the PS chain in P2 is shorter than that in P3, the adsorption rate of P2 is higher than that of P3. Therefore, more P2 than P3 molecules adsorb at the interface, leading to formation of a foam structure.
P2 formed a honeycomb structure and P3 formed a labyrinthine structure when the HAuCl4 concentration was further decreased to 5 × 10−5 mol L−1. The adsorption rates of the molecules play a key role in this case. Although the interaction between P3 molecules is stronger, there are only a small number of molecules at the interface. The wide open interface allows adsorbed molecules to organize themselves.
The length of the P2VP block also impacts the adsorption and self-assembly behavior of the block copolymers. Longer P2VP chains promote adsorption and enhance the interaction between molecules. While the PS blocks in P3 and P5 have the same length, the length of P2VP block is shorter in P3 than in P5. As shown in Fig. 2 and 4, different P2VP block lengths affect the microstructures. For example, P3 formed a foam structure when using the higher concentration aqueous HAuCl4 solution, while P5 formed both foam and planar films. When the concentration of HAuCl4 was decreased to 2 × 10−4 mol L−1, P3 formed a honeycomb structure, while P5 formed both foam and honeycomb structures.
In short, the polymer molecular structure affects the adsorption and interaction of the molecules, the organization of adsorbed molecules, and thus the formed microstructures. The microstructures result from synergistic effects of these factors.
We have investigated that foam and honeycomb structures appeared in the composite films of poly(t-butyl methacrylate)-block-poly(2-vinyl pyridine) (PtBMA-b-P2VP)/Ag formed at the liquid/liquid interface.16 We have also found that composite foam and honeycomb structures and nanobelts of poly(2-vinylpyridine)-b-polystyrene-b-poly(2-vinylpyridine) (P2VP-b-PS-b-P2VP)/Au were formed at the liquid/liquid interface under different conditions.17 These results, together with the results described in this paper suggest that amphiphilic block copolymers can form various micro- and nanostructures at the liquid/liquid interface under appropriate conditions. This is a universal phenomenon, which can be applied to fabricate various composite structures.
3.6. Catalytic activities
The reduction of 4-NA by KBH4 in aqueous solutions was used as a model reaction to evaluate the catalytic activities of the formed composite microstructures. Three thin layers formed by P3 at different HAuCl4 concentrations were used as catalysts. As shown in Fig. 6, the absorption peak of 4-NA centered at 380 nm weakened gradually with time, indicating that 4-NA was reduced to p-phenylenediamine in the presence of catalyst. It should be noted that the catalyst was just a thin layer formed under the lowest concentration of HAuCl4 in this case, whose morphology and structure were shown in Fig. 2(e, f) and 3(c). This result indicated the effective catalytic activity of the composite microstructure. The reduction of 4-NA under this condition is a pseudo first order reaction since the concentration of KBH4 is much higher than that of 4-NA. The apparent rate constant of the reaction was obtained from the slope of the ln(At/A0) ∼ t fitted line, where At and A0 are the intensities of the peak at 380 nm at time t and 0, respectively. The value was found to be 0.00764 min−1.To our surprise, the apparent rate constants were found to be 0.00732 and 0.00724 min−1 when the composite thin layers formed under the HAuCl4 concentrations of 2 × 10−4 and 1 × 10−3 mol L−1 were used as catalysts, which are close to each other, and close to the value obtained when using the thin layer formed using 5 × 10−5 mol L−1 HAuCl4 aqueous solution. As seen from Fig. 2, the thicknesses of these films are very different from one another. However, they exhibited similar apparent reaction rate for the reduction of 4-NA in aqueous solutions under the same conditions. This should be attributed to different structures of the films. The Au nanoclusters were incorporated in the polymer matrices in the foam films formed under higher HAuCl4 concentration, while the Au nanoclusters dispersed in the thin monolayers with labyrinth-like microstructure composed of nanowires. It was demonstrated that the diffusion of the reactants to the surface of the nanoparticles, adsorption and desorption of the reactants and products were essential for heterogeneous catalytic reactions.39 Undoubtedly, the thin layer formed using the lowest HAuCl4 concentration is the most favorable microstructure for the diffusion of 4-NA and KBH4 molecules among the three kinds of microstructures, although the amount of Au nanoclusters in this thin layer is greatly less than those in the other two microstructures. Therefore, the catalytic activities of the composite thin films are closely related to their morphologies and structures.
4. Conclusions
Composite microstructures of amphiphilic polymer PS-b-P2VP and gold nanoclusters were fabricated at the liquid/liquid interface of the polymer chloroform solution and HAuCl4 aqueous solution through an adsorption, combination and self-assembly process. Various microstructures, including foam structure composed of microcapsules, honeycomb structure, labyrinth-like structure composed of nanowires and fish net-like structure were fabricated under different conditions. The concentrations of the solutions and the polymer molecular structures greatly influence morphologies of the formed microstructures, because these factors affect the adsorption rate and intermolecular interactions of the polymer molecules. Other amphiphilic block copolymers, such as PtBMA-b-P2VP and P2VP-b-PS-b-P2VP, also self-assembled into such microstructures at the liquid/liquid interface under different conditions, suggesting the universality of this phenomenon. These composite films exhibited effective and morphology-dependent catalytic activities for heterogeneous catalytic reactions, such as the reduction of nitro-compounds in aqueous solutions.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21273133 and 21033005).References
- X. Wang, M. Goswami, R. Kumar, B. G. Sumpter and J. Mays, Soft Matter, 2012, 8, 3036–3052 RSC.
- S. E. Mastroianni and T. H. Epps III, Langmuir, 2013, 29, 3864–3878 CrossRef CAS PubMed.
- M. Luo and T. H. Epps III, Macromolecules, 2013, 46, 7567–7579 CrossRef CAS.
- J. Y. Park and R. C. Advincula, Soft Matter, 2011, 7, 9829–9843 RSC.
- L. Zhao and Z. Lin, Soft Matter, 2011, 7, 10520–10535 RSC.
- C. Sinturel, M. Vayer, M. Morris and M. A. Hillmyer, Macromolecules, 2013, 46, 5399–5415 CrossRef CAS.
- J. Wang, W. Li and J. Zhu, Polymer, 2014, 55, 1079–1096 CrossRef CAS PubMed.
- M. J. Pavan and R. Shenhar, J. Mater. Chem., 2011, 21, 2028–2040 RSC.
- C. N. R. Rao, G. U. Kulkarni, V. V. Agrawal, U. K. Gautam, M. Ghosh and U. Tumkurkar, J. Colloid Interface Sci., 2005, 289, 305–318 CrossRef CAS PubMed.
- B. Liu, D.-J. Qian, H.-X. Huang, T. Wakayama, S. Hara, W. Huang, C. Nakamura and J. Miyake, Langmuir, 2005, 21, 5079–5084 CrossRef CAS PubMed.
- S. Yang, C.-F. Wang and S. Chen, J. Am. Chem. Soc., 2011, 133, 8412–8415 CrossRef CAS PubMed.
- C. Zhou, N. Chen, J. Yang, H. Liu and Y. Li, Macromol. Rapid Commun., 2012, 33, 688–692 CrossRef CAS PubMed.
- Z. Sun, T. Feng and T. P. Russell, Langmuir, 2013, 29, 13407–13413 CrossRef CAS PubMed.
- D. Wang, H. Ma, C. Chu, J. Hao and H.-G. Liu, J. Colloid Interface Sci., 2013, 402, 75–85 CrossRef CAS PubMed.
- Y. Liu, L. Chen, Y. Geng, Y.-I. Lee, Y. Li, J. Hao and H.-G. Liu, J. Colloid Interface Sci., 2013, 407, 225–235 CrossRef CAS PubMed.
- C. Chu, D. Wang, H. Ma, M. Yu, J. Hao and H.-G. Liu, Mater. Chem. Phys., 2013, 142, 259–267 CrossRef CAS PubMed.
- K. Shang, Y. Geng, X. Xu, C. Wang, Y.-I. Lee, J. Hao and H.-G. Liu, Mater. Chem. Phys., 2014, 146, 88–98 CrossRef CAS PubMed.
- Z. Yang, Z. Wang, X. Yao and Y. Wang, Langmuir, 2012, 28, 3011–3017 CrossRef CAS PubMed.
- C.-S. Lim, S. I. Seok and S. H. Im, J. Mater. Chem., 2012, 22, 8772–8774 RSC.
- J. H. Youk, M.-K. Park, J. Locklin, R. Advincula, J. Yang and J. Mays, Langmuir, 2002, 18, 2455–2458 CrossRef CAS.
- J. Polleux, M. Rasp, I. Louban, N. Plath, A. Feldhoff and J. P. Spatz, ACS Nano, 2011, 5, 6355–6364 CrossRef CAS PubMed.
- L. Wang, F. Montagne, P. Hoffmannb and R. Pugina, Chem. Commun., 2009, 3798–3800 RSC.
- M. Aizawa and J. M. Buriak, Chem. Mater., 2007, 19, 5090–5101 CrossRef CAS.
- L. Wang, F. Montagne, P. Hoffmann, H. Heinzelmann and R. Pugin, J. Colloid Interface Sci., 2011, 356, 496–504 CrossRef CAS PubMed.
- Y. Liu, C. Lor, Q. Fu, D. Pan, L. Ding, J. Liu and J. Lu, J. Phys. Chem. C, 2010, 114, 5767–5772 CAS.
- C. M. Grozea, N. Gunari, J. A. Finlay, D. Grozea, M. E. Callow, J. A. Callow, Z.-H. Lu and G. C. Walker, Biomacromolecules, 2009, 10, 1004–1012 CrossRef CAS PubMed.
- Y. Lu, H. Xia, G. Zhang and C. Wu, J. Mater. Chem., 2009, 19, 5952–5955 RSC.
- J. Yin, X. Yao, J.-Y. Liou, W. Sun, Y.-S. Sun and Y. Wang, ACS Nano, 2013, 7, 9961–9974 CrossRef CAS PubMed.
- J. J. Chiu, B. J. Kim, E. J. Kramer and D. J. Pine, J. Am. Chem. Soc., 2005, 127, 5036–5037 CrossRef CAS PubMed.
- B. J. Kim, J. Bang, C. J. Hawker, J. J. Chiu, D. J. Pine, S. G. Jang, S.-M. Yang and E. J. Kramer, Langmuir, 2007, 23, 12693–12703 CrossRef CAS PubMed.
- X. Li, S. Zhao, S. Zhang, D. H. Kim and W. Knoll, Langmuir, 2007, 23, 6883–6888 CrossRef CAS PubMed.
- S. G. Jang, A. Khan, C. J. Hawker and E. J. Kramer, Macromolecules, 2012, 45, 1553–1561 CrossRef CAS.
- B. Chung, M. Choi, M. Ree, J. C. Jung, W. C. Zin and T. Chang, Macromolecules, 2006, 39, 684–689 CrossRef CAS.
- G. Wen, B. Chung and T. Chang, Polymer, 2006, 47, 8575–8582 CrossRef CAS PubMed.
- Y.-S. Seo, K. S. Kim, A. Galambos, R. G. H. Lammertink, G. J. Vancso, J. Sokolov and M. Rafailovich, Nano Lett., 2004, 4, 483–486 CrossRef CAS.
- G. Wen, J. Phys. Chem. B, 2010, 114, 3827–3832 CrossRef CAS PubMed.
- L.-J. Chen, H. Ma, K. Chen, H.-R. Cha, Y.-I. Lee, D.-J. Qian, J. Hao and H.-G. Liu, J. Colloid Interface Sci., 2011, 362, 81–88 CrossRef CAS PubMed.
- L. Lin, K. Shang, X. Xu, C. Chu, H. Ma, Y.-I. Lee, J. Hao and H.-G. Liu, J. Phys. Chem. B, 2011, 115, 11113–11118 CrossRef CAS PubMed.
- H. Ma, Y. Geng, Y.-I. Lee, J. Hao and H.-G. Liu, J. Colloid Interface Sci., 2011, 394, 223–230 CrossRef PubMed.
- H.-L. Zhang, S. D. Evans, J. R. Henderson, R. E. Miles and T. Shen, J. Phys. Chem. B, 2003, 107, 6087–6095 CrossRef.
- J.-P. Sylvestre, S. Poulin, A. V. Kabashin, E. Sacher, M. Meunier and J. H. T. Luong, J. Phys. Chem., 2004, 108, 16864–16869 CrossRef CAS.
- A. Kumar, S. Mandal, S. P. Mathew, P. R. Selvakannan, A. B. Mandale, R. V. Chaudhari and M. Sastry, Langmuir, 2002, 18, 6478–6482 CrossRef CAS.
- S. Kobayashi and R. Akiyama, Chem. Commun., 2003, 449–460 RSC.
| This journal is © The Royal Society of Chemistry 2015 |