Lipopeptide biosurfactant from Bacillus clausii BS02 using sunflower oil soapstock: evaluation of high throughput screening methods, production, purification, characterization and its insecticidal activity†
Abstract
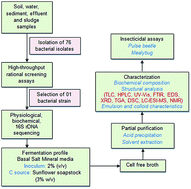
A total of 76 bacterial isolates were screened for biosurfactant activity using 16 different high throughput screening methods. Based on phenotypic characteristics, 16S rDNA sequencing and FAME profiling, isolate BS02, identified as Bacillus clausii BS02 produced a biosurfactant (2.6 g l−1) when grown in basal salts mineral medium with sunflower oil soapstock (3% w/v) at 30 °C and 120 rpm after 42 h. The cell free broth showed 82% emulsification activity, critical micelle concentration of 45 mg l−1 and reduced the surface tension from 69.07 mN m−1 to 30 mN m−1. Acid precipitated and methanol extracted crude biosurfactant had 64.3%, 28%, and 4.5% (w/w) of protein, lipid, and carbohydrate, respectively. The crude biosurfactant exhibited stability with oils, fats and non-aqueous phase liquids. Fourier transform infrared (FT-IR) spectroscopy, energy dispersive X-ray spectroscopy (EDX), and nuclear magnetic resonance spectroscopy (NMR) revealed the functional groups and bonds; X-ray diffraction (XRD) and thermogravimetry (TG) showed the surface nature and thermostability of the biosurfactant, respectively. LC-ESI-MS identified the crude biosurfactant as a surfactin lipopeptide. A dose-dependent mortality against C. chinensis and M. hirsutus was observed using the crude biosurfactant, with a LC50 of 50 μg ml−1, indicating the first evidence of its insecticidal activity against pulse beetles and mealybugs.

 Please wait while we load your content...
Please wait while we load your content...