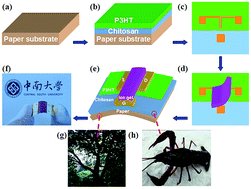
Reusable and renewable electronics is an emerging field aimed at the development of environmentally safe, disposable and biocompatible devices. Here, we demonstrate the fabrication of flexible poly(3-hexylthiophene) (P3HT) field-effect transistors (FETs) on cellulose paper with biopolymer chitosan smoothing layers and efficient reusable ion gel gate dielectrics. The high specific capacitance of ion gels led to the formation of an electric-double-layer (EDL) at the channel/dielectric interface that plays a critical role in modulating the currents of the devices. Reasonable electrical characteristics and mechanical flexibility were observed. Transistors with an operating voltage as low as 2 V, a source drain current of up to ∼1 mA, a current on/off ratio of 3–4 orders of magnitude and a large field-effect mobility of ∼0.97 cm2 V−1 s−1 have been obtained. Especially, the laminated ion gels can be in situ peeled off and reused in other FETs and the device performance is not degenerated obviously with repeated use. The unique flexibility of the FETs on paper with reusable ion gel dielectrics manifests their applications as the next generation of throwaway electronics.