High performance epoxy protective coatings incorporated with polyaniline nanowires using cardanol-based phenalkamine as the curing agent
Di Chuabcd,
Jixiao Wang*abcd,
Yufeng Hanabcd,
Qiang Maabcd and
Zhi Wangabcd
aChemical Engineering Research Center, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, PR China
bTianjin Key Laboratory of Membrane Science and Desalination Technology, Tianjin University, Tianjin 300072, PR China
cState Key Laboratory of Chemical Engineering, Tianjin University, Tianjin 300072, PR China
dCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin 300072, PR China
First published on 9th January 2015
Abstract
To investigate the performance of cardanol-based phenalkamine as curing agent, epoxy protective coatings were prepared. Different weight contents of polyaniline nanowires were also incorporated to improve the protective performance of the coatings. The wettability and the adhesion strength of the coatings were investigated. The prepared coatings were tested by immersing the samples in 12 wt% NaCl solution at 95 °C and the electrochemical impedance spectroscopy technique was used to evaluate the performance of the coatings. The experimental results indicate that the epoxy protective coatings cured by cardanol-based phenalkamine provide excellent protection for mild carbon steel. The coating containing 2 wt% polyaniline nanowires exhibits the best corrosion resistance performance and its impedance modulus at 0.01 Hz is close to 1 × 1011 Ohm cm2 after 60 days of immersion. The passive properties of polyaniline nanowires were characterized by scanning electron microscopy and X-ray photoelectron spectroscopy.
1. Introduction
Corrosion of metals not only causes great economic losses, but leads to structural failures that bring catastrophic consequences to humans and the environment. The most commonly used and convenient method for metal protection is the application of coatings, especially organic coatings. Epoxy resin has advantages over other synthetic resins as the matrix of protective coatings owing to its superior chemical and corrosion resistance, excellent adhesion, and low shrinkage.1 The curing agents initiating cross linking of epoxy also have a significant effect on the coating's performance. Polyamides and phenalkamines prepared by reacting amines with phenol and formaldehyde are the most important kinds of curing agents at room temperature.2Using of renewable sources from the nature products should be a sustainable and promising method in the manufacture of polymers. Cardanol, obtained by distillation of cashew nut shell liquid, an agricultural by-product of the cashew industry, containing a phenolic moiety with an unsaturated 15-carbon side chain, can replace phenol in many applications.3–5 Compared with conventional phenalkamines, phenalkamines synthesized from cardanol exhibit flexibility due to “internal plasticization” of the 15-carbon side chain. The aliphatic side chain also imparts hydrophobic character, making the coating water repellent.6,7
Significant efforts have been made towards corrosion prevention by using conductive polymers (CPs). Until now, CPs including polyaniline (PANI), polypyrrole, polythiophene and their derivatives have been intensively investigated as inhibitive additives.8–12 The corrosion protection of conductive polymers may be attributed to their barrier effect and their facilitating formation of dense passive films.13–15 PANI, because of its simple synthesis, low cost monomer, tunable properties, and high stability and environmental benefit, is the most studied conductive polymer.16,17 The reversible conversion of PANI among its different oxidation states could facilitate formation of a passive film on the metal surface to protect the metal from further corrosion.17–19
Here, high performance epoxy protective coatings incorporated with PANI nanowires cured by cardanol-based phenalkamine were prepared for carbon steel protection. The influence of PANI nanowire content on coating's protective performance was investigated. The coatings were tested by immersion the samples in 12 wt% NaCl solution at 95 °C for 60 days. The corrosion resistance performance of the coatings was investigated by electrochemical impedance spectroscopy (EIS) and the results were simulated with electrochemical simulation models. The experimental results might give some insights on the design of high-performance epoxy coatings in heavy duty industrial and marine applications.
2. Experimental section
2.1. Materials
The epoxy (diglycidyl ether of bisphenol A, 185–210 g eq.−1) serving as the matrix component was purchased from Jiangsu Sanmu Group, China. The phenalkamine curing agent synthesized from cardanol (MD1041, 270–310 mg KOH per g) was obtained from Shanghai Meidong Biomaterials Co., China. The conventional phenalkamine curing agent (T31, 460–480 mg KOH/g) was purchased from Tianjin Yanhai Reagent Ltd. Co., China. Methyl ethyl ketone (MEK) applied as solvent was purchased from Tianjin Jiangtian Reagent Ltd. Co., China. PANI nanowires were purchased from Tianjin Advanced materials Ltd. Co., China, which was prepared electrochemically. All the raw chemicals were used directly as received without purification.Mild steel plates (Q235, C: 0.17–0.24%; Si: 0.17–0.37%; Mn: 0.35–0.65%; S: <0.030%; P: <0.030%) were used as the substrates for the corrosion tests. Prior to application, the plates were grinded successively with 400#, 800#, 1200#, and 2000# abrasive papers, followed by a thorough rinse with deionized water and anhydrous ethanol, and then air dried.
2.2. Preparation of coatings
PANI/phenalkamine blend was prepared by milling calculated amount of PANI nanowires with the phenalkamine in methyl ethyl ketone. Epoxy resin was added into methyl ethyl ketone to form an epoxy solution, and then mixed the blend of PANI/phenalkamine. The mixture was brushed onto the pretreated mild steel plates. All the coatings were cured at room temperature for 7 days before testing. The thickness of the coatings was about 150 (±10) μm. By adjusting the amount of PANI nanowires used, coatings containing 1 wt%, 2 wt% and 3 wt% PANI nanowires were prepared. As a reference, an epoxy coating without PANI nanowires was also prepared under the same conditions.2.3. Characterizations
The morphology of the PANI nanowires and the coating surface were characterized by scanning electron microscopy (SEM) with a JEOL JSM-6700F microscope. The optical homogeneity of dispersions of PANI nanowires in the curing agent was examined using a HIROX digital microscope (KH-7700, Japan).The contact angle of the coatings was measured at ambient temperature on a Dataphysics OCA15EC contact-angle goniometer following the sessile drop method. Five measurements were performed on each sample and the values averaged.
The water absorption of the coatings was measured by gravimetric method. The dry samples (6 cm × 8 cm) prepared as described in Section 2.2 were weighed and then immersed in distilled water at room temperature. The samples were weighed periodically until the weight reached a constant value. The accuracy of the weight measurement is in the order of ±0.01 mg.
Pull-off adhesion tests were performed with PosiTest Adhesion Tester (PosiTest AT-M, Defelsko, USA) to measure the adhesion strength of the coatings. The dollies were glued vertically to the surface of the coating using an epoxy adhesive. After complete curing of the adhesive and removing the excess adhesive, the dollies were pulled off from the substrate. Three points were tested on each sample.
EIS tests were performed at different intervals of the immersion test in 12 wt% NaCl solution. The measurements were carried out on an electrochemical workstation (VersaSTAT, USA) at room temperature in a conventional three-electrode electrochemical cell. A graphite rod, a saturated calomel electrode and the coated sample with an exposure area of approximately 1.00 cm2 were used as the counter electrode, the reference electrode, and the working electrode, respectively. The cell was placed in a faraday cage to avoid Coulombs fields. The electrolyte used in the cell was also 12 wt% NaCl solution. The frequency ranged from 10 mHz to 100 kHz and the amplitude was 10 mV. The impedance data were analyzed and modeled into equivalent electrical circuits (EEC) using “ZSimpWin”.
The surface of the steel plate after peeling off the coating film was characterized using an X-ray photoelectron spectroscopy (XPS, PHI-1600). XPS analysis was performed using Mg Kα as the radiation source, and the spectra were taken with the electron emission angle at 45°. Spectra of Fe 2p were recorded.
3. Results and discussion
3.1. Characterization of PANI nanowires and their dispersibility
The SEM image of PANI nanowires used in this research was presented in Fig. 1(a). The figure clearly shows that the PANI nanowires were about 100 nm in diameter and several microns in length. The dispersibility of the PANI nanowires in the curing agent was characterized using optical microscopy and was shown in Fig. 1(b). The optical microscopy image shows that when the content of PANI nanowires is 2 wt%, PANI can be well dispersed, as particles of typically less than 5 μm in diameter with little aggregation, in the curing agent. | ||
| Fig. 1 (a) SEM image of the PANI nanowires, (b) optical micrograph of PANI/phenalkamine blend with PANI nanowires content of 2 wt%. | ||
3.2. Wettability of the coatings
The wettability of the coatings was evaluated by contact angle measurement and water absorption measurement. Static contact angles of the coating without PANI cured by T31 and the coatings containing 0 wt%, 1 wt%, 2 wt% and 3 wt% PANI nanowires cured by MD1041 were measured. The photos are presented in Fig. 2. The contact angles of the coatings are 48°, 56°, 58°, 60°, and 62°, respectively. Compared with conventional phenalkamine, phenalkamine synthesized from cardanol cured coatings have large contact angles. This contact angle increase demonstrates that the presence of the long aliphatic side chain in cardanol improves the coating's water repellency. The slight contact angle increase with the addition of PANI nanowires might be ascribed to the increase of the surface roughness.17,20 | ||
| Fig. 2 Contact angles for the coatings cured by (a) T31, (b) MD1041, (c) MD1041 with 1 wt% PANI, (d) MD1041 with 2 wt% PANI, and (e) MD1041 with 3 wt% PANI. | ||
The results of the water absorption measurements are presented in Fig. 3, from which we can see that the coating cured by T31 absorbs more water than the coatings cured by MD1041 and the incorporation of PANI reduces the water absorption. These results agree well with that of the contact angle measurement results. Since high water proofing property of the coatings will delay the fading, chalking, cracking, and flaking process, the corrosion protection performance of the coatings can be improved to some degree by choosing the phenalkamine synthesized from cardanol as curing agent and incorporating PANI nanowires.
3.3. Adhesion strength of the coatings
As we can see from the results of the adhesion tests shown in Table 1, the adhesion strength of the epoxy coating slightly increases when PANI nanowires are added to the epoxy coating. The average adhesion strength of the epoxy coating without PANI nanowires is 3.87 MPa, while it increases to 4.21 MPa, 4.45 MPa, and 4.63 MPa with 1 wt%, 2 wt% and 3 wt% PANI nanowires, respectively. Superior adhesion strength of the coatings may result from the reduced total free volume after incorporating nanomaterials.21 The results clearly demonstrate the positive effect of the presence of PANI nanowires on the adhesion strength of the epoxy coatings.| Samples | Pure epoxy | Epoxy + 1 wt% PANI | Epoxy + 2 wt% PANI | Epoxy + 3 wt% PANI |
|---|---|---|---|---|
| Pull-off stress (MPa) | 3.87 | 4.21 | 4.45 | 4.63 |
3.4. EIS studies and equivalent electrical circuit evaluation
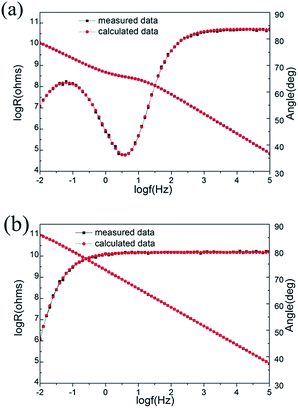
The results of the EIS measurements of different coatings, as Bode plots, after different immersion time in 12 wt% NaCl solution at 95 °C are presented in Fig. 4. Corrosion, a process of losing electrons of metals, can be presented as an electron orientated moving process. In EIS measurement, the lower the applied frequency is, the closer the current to the corrosion current, which represent the corrosion rate. So the impedance modulus at low frequencies, such as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R at 0.01 Hz may be apparently used to estimate the protective performance of coatings.22
R at 0.01 Hz may be apparently used to estimate the protective performance of coatings.22
 | ||
| Fig. 4 Bode plots at different immersion time in 12 wt% NaCl solution at 95 °C for (a) pure epoxy, (b) epoxy with 1 wt% PANI, (c) epoxy with 2 wt% PANI, and (d) epoxy with 3 wt% PANI coatings. | ||
In the initial days of immersion, the Bode plots for epoxy coating without PANI nanowires and coatings with PANI nanowires, are almost straight lines with the slope approximate to −1, indicating the coatings act almost as perfect capacitors.23 After 26 days of immersion, the Bode plots are no longer straight lines for all coatings. Correspondingly, all the Nyquist diagrams are characterized by two incomplete capacitive loops, a smaller one at high frequency range, which is attributed to the coating performance, followed by a larger one at low frequency range, which is related to the processes occurring underneath the coating.24 In order to clearly present the capacitive loop at high frequencies, the Nyquist plots without the last few points at low frequencies are shown in Fig. 5.
 | ||
| Fig. 5 Nyquist plots after different days of immersion in 12 wt% NaCl solution for (a) pure epoxy, (b) epoxy with 1 wt% PANI, (c) epoxy with 2 wt% PANI, and (d) epoxy with 3 wt% PANI coatings. | ||
For the epoxy coating without PANI nanowires, once water and/or electrolyte penetrate through the coating, the corrosion process on the surface of the substrate will occur, which results in decreasing of impedance modulus in the entire later stage of immersion (Fig. 4(a)). For coatings containing PANI nanowires, the Bode plots retrieve to straight lines and only one time constant remains in later immersion, and the impedance modulus at low frequencies even higher than that of initial values (Fig. 4(b) and (d)). These are ascribed to the formation of protective passive films on the metal surface induced by PANI nanowires, which can avoid the access of corrosive media to the metal surface, retarding the corrosion of metal.25,26
It is noted that for the coatings containing PANI nanowires, the period to retrieve high impedance along with the disappearance of the second time constant is different. The EIS measurement results show that after 30 days of immersion, the coatings containing 1 wt% and 3 wt% PANI nanowires still featured with two time constants. But the second time constant disappears at 40 days in both cases (Fig. 5(b) and (d)). Comparatively, when the content of PANI nanowires is 2 wt%, only one time constant can be observed at 30 days (Fig. 5(c)) and the impedance modulus is higher than that of the other cases. In the period of 60 days immersion, the coating containing 3 wt% PANI start to deteriorate and two time constants can be detected again (Fig. 5(d)), which may result from the low dispersibility of PANI nanowires. The Bode plots of the coatings with 1 wt% and 2 wt% PANI nanowires are still straight lines (Fig. 4(b) and (c)). The impedance modulus at 0.01 Hz of the coating containing 2 wt% PANI nanowires is the highest of all the coatings, reaching 9.58 × 1010 Ohm cm2. The superior corrosion resistance performance of this coating system indicates the appropriate amount of PANI nanowires is necessary and PANI nanowires content of 2 wt% is the optimal in this study.
The coating containing polyaniline–TiO2 composite showed impedance modulus of 107 Ohm cm2 after 60 days room temperature immersion in 3 wt% NaCl solution.27 Epoxy/polyamide coatings containing nano-ZnO and nano-Al2O3 had impedance modulus of around 109 Ohm cm2 after 60 days room temperature immersion in 3.5 wt% NaCl solution.28,29 Compared with these formulations reported, the protective property of the epoxy/cardanol-based phenalkamine coating containing PANI nanowires is excellent.
The equivalent electrical circuit (EEC) fitting method is employed to compare the EIS data of the coatings and analyze the effect of PANI nanowires. The values of the elements in the circuits can provide information concerning the protective performance of coatings as they degrade. The electrochemical behavior of the epoxy coating can be modeled with the EEC presented in Fig. 6(a). Constant phase elements (CPE) are employed to replace all capacitance elements in EECs to obtain more precious fitting results. Rs, Rc, CPEc, CPEd and Rct refer to solution resistance, coating resistance, coating capacitance, double layer capacitance and charge transfer resistance, respectively. Fig. 7(a) shows the fitting results for both the impedance modulus and phase angle plots at 60 days.
The fitting of the EIS data of the coatings containing 1 wt% and 2 wt% PANI nanowires after 60 days immersion was performed using a simple EEC model which is a parallel RQ circuit in series with the solution resistance, as shown in Fig. 6(b). The fitting results for both the impedance modulus and phase angle plots of the coating with 2 wt% PANI nanowires after 60 days immersion are shown in Fig. 7(b). Both modeling results match well with the experimental data. As the coatings containing 3 wt% PANI nanowires start to degrade after 60 days immersion and two time constants are presented, the EEC chosen to fit the EIS data is the one showed in Fig. 6(a). The coating resistance (Rc) is close related to the porosity and degradation of the coating, and coatings with high resistance values (>107 Ohm cm2) have been found to offer corrosion protection for steels.30–32 In fact, the corrosion process is not only affected by coating resistance, but by charge transfer resistance, which may be governed by passive films. The higher the charge transfer resistance is, the lower the corrosion rate is.33 The resistance values of the EEC models of all the coating systems after 60 days immersion are presented in Table 2.
| Samples | Pure epoxy | Epoxy + 1 wt% PANI | Epoxy + 2 wt% PANI | Epoxy + 3 wt% PANI |
|---|---|---|---|---|
| Rc (Ohm cm2) | 2.92 × 108 | 9.47 × 1010 | 1.76 × 1011 | 2.70 × 108 |
| Rct (Ohm cm2) | 3.87 × 1010 | — | — | 7.28 × 1010 |
As shown in Table 2, the values of the coating resistance of all the coatings are still high even after being immersed for 60 days. The highest value of coating resistance is achieved by the coating with 2 wt% PANI nanowires, followed by the coating with 1 wt% PANI nanowires. Though the EEC with two constant phase elements are used to fit the EIS data of the epoxy coating and the coating with 3 wt% PANI nanowires, their charge transfer resistances, which are directly related to the rate of the corrosion reactions at the coating/metal interface are high. The experimental result indicates these two coatings also possess moderately high corrosion protection ability. The fitting results confirm that epoxy coatings cured by cardanol phenalkamine have outstanding corrosion resistance and the addition of PANI nanowires can further improve its protective performance.
3.5. Surface analysis
The morphology of the inner surface of the pure epoxy coating and the coating with 2 wt% PANI nanowires is shown in Fig. 8. It can be seen that the inner surface of both coatings before immersion exhibit obvious traces which were formed by rubbing the steel plates with sandpaper (Fig. 8(a) and (b)). After 60 days immersion, the inner surface of the pure epoxy coating becomes coarse, which may attributable to the formation of some corrosion products, though the EIS study reveals good protection is provided by the coating (Fig. 8(c)). For the coating with 2 wt% PANI nanowires, large particles are observed, which could be iron oxide complex induced by PANI (Fig. 8(d)).30Fig. 9 shows XPS of Fe 2p spectra for the corresponding steel surface. For pure coating, the peak observed at binding energy of 710.56 and 724.21 correspond to Fe 2p3/2 and Fe 2p1/2 reveals that the sample has phase of Fe3+.34 The peaks are observed at 711.06 and 724.64 for coating with 2 wt% PANI. The peaks shift to high binding energy for Fe3O4 due to the appearance of Fe2+ (2p3/2) and Fe2+ (2p1/2).35 These confirm the formation of Fe3O4 for coating with 2 wt% PANI, and the satellite feature observed at around 719 suggests the presence of an extent of Fe2O3.25,36 Combining with both SEM and XPS results, we conclude that PANI has strong interaction with steel and a protective oxide layer (Fe2O3/Fe3O4) can be formed on the steel surface. Thus, the coating containing PANI has higher impedance and can maintain effective for longer time than pure epoxy coating.
 | ||
| Fig. 9 XPS Fe 2p spectra for steel surface of (a) pure epoxy, (b) epoxy with 2 wt% PANI after 60 days immersion. | ||
4. Conclusions
High performance epoxy protective coatings PANI nanowires cured by phenalkamine synthesized from cardanol were prepared and characterized. The immersion test and the EIS measurements reveal that the coatings can protect the mild steel from corrosion in the environment of high temperature and high salinity. Incorporation of PANI nanowires helps improve the water repellency and adhesion strength of the coatings, but higher amount of PANI nanowires cannot be dispersed well, resulting in less effective protective performance. The coating with 2 wt% PANI nanowires exhibits the best corrosion resistance performance. Its impedance modulus at 0.01 Hz after 60 days immersion is the highest among all the prepared coatings, which is close to 1 × 1011 Ohm cm2. To the best of our knowledge, the protective performance is superior to that of the reported coatings containing popular nanomaterials as additives.Acknowledgements
This work was supported by Public Science and Technology Research Funds Projects of Ocean (no. 201405013-5), the Program of Introducing Talents of Discipline to Universities (no. B06006), National Natural Science Foundation of China (20836006), National High Technology Research and Development Program of (2012AA03A611), and SKL-ChE-12T12.Notes and references
- P. A. Sørensen, S. Kiil, K. Dam-Johansen and C. E. Weinell, J. Coat. Technol. Res., 2009, 6, 135–176 CrossRef.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J. P. Pascault, Chem. Rev., 2014, 114, 1082–1115 CrossRef CAS PubMed.
- C. Voirin, S. Caillol, N. V. Sadavarte, B. V. Tawade, B. Boutevin and P. P. Wadgaonkar, Polym. Chem., 2014, 5, 3142 RSC.
- V. S. Balachandran, S. R. Jadhav, P. K. Vemula and G. John, Chem. Soc. Rev., 2013, 42, 427–438 RSC.
- B. Lochab, S. Shukla and I. K. Varma, RSC Adv., 2014, 4, 21712 RSC.
- S. Mohapatra and G. B. Nando, RSC Adv., 2014, 4, 15406 RSC.
- J. M. Raquez, M. Deléglise, M. F. Lacrampe and P. Krawczak, Prog. Polym. Sci., 2010, 35, 487–509 CrossRef CAS PubMed.
- Z. Tian, H. Yu, L. Wang, M. Saleem, F. Ren, P. Ren, Y. Chen, R. Sun, Y. Sun and L. Huang, RSC Adv., 2014, 4, 28195 RSC.
- D. Kowalski, M. Ueda and T. Ohtsuka, J. Mater. Chem., 2010, 20, 7630 RSC.
- N. K. Rawat, A. Ghosal and S. Ahmad, RSC Adv., 2014, 4, 50594–50605 RSC.
- E. Armelin, Á. Meneguzzi, C. A. Ferreira and C. Alemán, Surf. Coat. Technol., 2009, 203, 3763–3769 CrossRef CAS PubMed.
- C. Xing, Z. Zhang, L. Yu, L. Zhang and G. A. Bowmaker, RSC Adv., 2014, 4, 32718 RSC.
- E. Armelin, R. Oliver, F. Liesa, J. I. Iribarren, F. Estrany and C. Alemán, Prog. Org. Coat., 2007, 59, 46–52 CrossRef CAS PubMed.
- A. F. Baldissera and C. A. Ferreira, Prog. Org. Coat., 2012, 75, 241–247 CrossRef CAS PubMed.
- E. Armelin, R. Pla, F. Liesa, X. Ramis, J. I. Iribarren and C. Alemán, Corros. Sci., 2008, 50, 721–728 CrossRef CAS PubMed.
- H. Zhang, J. Wang, Z. Wang, F. Zhang and S. Wang, Synth. Met., 2009, 159, 277–281 CrossRef CAS PubMed.
- H. Zhang, J. Wang, X. Liu, Z. Wang and S. Wang, Ind. Eng. Chem. Res., 2013, 52, 10172–10180 CrossRef CAS.
- W.-K. Lu, R. L. Elsenbaumer and B. Wessling, Synth. Met., 1995, 71, 2163–2166 CrossRef CAS.
- J. L. Lu, N. J. Liu, X. H. Wang, J. Li, X. B. Jing and F. S. Wang, Synth. Met., 2003, 135–136, 237–238 CrossRef CAS.
- T. Darmanin and F. Guittard, Prog. Polym. Sci., 2014, 39, 656–682 CrossRef CAS PubMed.
- X. Shi, T. A. Nguyen, Z. Suo, Y. Liu and R. Avci, Surf. Coat. Technol., 2009, 204, 237–245 CrossRef CAS PubMed.
- B. R. Hinderliter, S. G. Croll, D. E. Tallman, Q. Su and G. P. Bierwagen, Electrochim. Acta, 2006, 51, 4505–4515 CrossRef CAS PubMed.
- M. Nematollahi, M. Heidarian, M. Peikari, S. Kassiriha, N. Arianpouya and M. Esmaeilpour, Corros. Sci., 2010, 52, 1809–1817 CrossRef CAS PubMed.
- E. Akbarinezhad, M. Ebrahimi and H. Faridi, Prog. Org. Coat., 2009, 64, 361–364 CrossRef CAS PubMed.
- Y. Chen, X. Wang, J. Li, J. Lu and F. Wang, Corros. Sci., 2007, 49, 3052–3063 CrossRef CAS PubMed.
- M. Bagherzadeh, F. Mahdavi, M. Ghasemi, H. Shariatpanahi and H. Faridi, Prog. Org. Coat., 2010, 68, 319–322 CrossRef CAS PubMed.
- S. Sathiyanarayanan, S. S. Azim and G. Venkatachari, Electrochim. Acta, 2007, 52, 2068–2074 CrossRef CAS PubMed.
- B. Ramezanzadeh, M. M. Attar and M. Farzam, Prog. Org. Coat., 2011, 72, 410–422 CrossRef CAS PubMed.
- S. Sharifi Golru, M. M. Attar and B. Ramezanzadeh, Prog. Org. Coat., 2014, 77, 1391–1399 CrossRef CAS PubMed.
- F. Chen and P. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 2694–2702 CAS.
- W.-G. Ji, J.-M. Hu, L. Liu, J.-Q. Zhang and C.-N. Cao, Surf. Coat. Technol., 2007, 201, 4789–4795 CrossRef CAS PubMed.
- S. Sathiyanarayanan, S. Muthkrishnan and G. Venkatachari, Electrochim. Acta, 2006, 51, 6313–6319 CrossRef CAS PubMed.
- T. C. T. P. Li and J. Y. Lee, Synth. Met., 1997, 88, 237–242 CrossRef.
- J. Gong, K. Yao, J. Liu, Z. Jiang, X. Chen, X. Wen, E. Mijowska, N. Tian and T. Tang, J. Mater. Chem. A, 2013, 1, 5247 CAS.
- W. S. Lu, Y. H. Shen, A. J. Xie and W. Q. Zhang, J. Magn. Magn. Mater., 2010, 322, 1828–1833 CrossRef CAS PubMed.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |