Facile fabrication of highly flexible graphene paper for high-performance flexible lithium ion battery anode†
Abstract
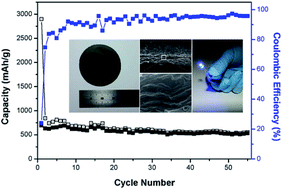
Freestanding paper-like materials prepared from chemically derived graphene have considerable potential as a carbon-based electrode in high-performance flexible energy storage devices. Herein, a highly flexible graphene paper (GP) assembled from graphene nanoplatelets (GNPs) with the aid of graphene oxides (GOs) is reported for a high-performance, binder- and conducting additive-free anode in lithium-ion batteries (LIBs). In contrast to previous reports on GPs based on a flow-directed assembly of graphene sheets, this GNP/GO paper exhibited a highly wrinkled and disordered morphology. When the GNP/GO paper was applied as a LIB anode, it showed a high specific capacity of 694 mA h g−1 and high rate performance. Furthermore, a pouch-type flexible LIB using the GNP/GO paper also showed a stable cycling behavior and practical performance. This GNP/GO paper electrode prepared using a simple, yet effective assembly of graphene derivatives, is highly promising for the fabrication of flexible energy storage devices.

 Please wait while we load your content...
Please wait while we load your content...