Li9V3(P2O7)3(PO4)2 nanotubes fabricated by a simple molten salt approach with excellent cycling stability and enhanced rate capability in lithium-ion batteries†
Xue Miaoab,
Chun Liab,
Wei Chu*c,
Ping Wuab and
Dong Ge Tong*ab
aMineral Resources Chemistry Key Laboratory of Sichuan Higher Education Institutions, College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China. E-mail: tongdongge@163.com; Fax: +86 28 8407 9074
bState Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Chengdu University of Technology, Chengdu 610059, China
cCollege of Chemical Engineering, Sichuan University, Chengdu 610065, China. E-mail: chuwei1965@foxmail.com; Fax: +86 28 8540 3397
First published on 21st November 2014
Abstract
Li9V3(P2O7)3(PO4)2 nanotubes were prepared on large scale by a simple molten salt route without the use of a surfactant as a template and the nanotubes exhibited excellent cycling stability in addition to enhanced rate capability as cathode materials for lithium-ion batteries.
Introduction
As cathode materials for rechargeable lithium-ion batteries (LIBs), Li9V3(P2O7)3(PO4)2 is of high fundamental and technological interest because of its high theoretical specific capacity (173.5 mA h g−1), its safety and its low cost.1 However, the rate performance of Li9V3(P2O7)3(PO4)2 is restricted by sluggish electron and lithium-ion transport kinetics.1 To address this problem, attention has been given to coating with conducting layers1c,f and to doping with isovalent ions.1d,g–i However, the unmodified and modified Li9V3(P2O7)3(PO4)2 materials produced to date do not comply with the demands of the practical application of lithium-ion batteries.One-dimensional (1D) nanostructured electrode materials such as nanowires, nanorods, nanobelts, and nanotubes have attracted much attention because of their higher capacity and stability.2 These properties come from their limited crystal dimensions and very high surface-to-bulk ratios.3 For example, Lou's group prepared single-crystal nanoneedle arrays of NiCo2O4 with very high capacitance and excellent cycling stability.2f Gao et al. reported that ordered WO3 nanowire arrays had high capacity and excellent rate capabilities as electrode materials in lithium-ion batteries.2g Although many 1D nanostructured materials have been prepared to date,4 few reports exist detailing the fabrication of 1D Li9V3(P2O7)3(PO4)2 nanostructures. This may come from the complex structural chemistry of Li9V3(P2O7)3(PO4)2 such as the many valences of vanadium oxide and sensitivity toward O2 pressure, etc.1 The preparation of 1D nanostructures of trigonal phases is difficult because of 3D isotropic growth. Templates (including soft and hard templates)5 are often required as are accessional environmental conditions (such as an ultrasonic field, etc.) to assist in fabrication.6 Therefore, the development of simple routes to prepare 1D nanostructures of trigonal phase Li9V3(P2O7)3(PO4)2 is important and challenging and it may lead to an improvement in electrochemical performance.
We thus introduce a molten salt method for the fabrication of Li9V3(P2O7)3(PO4)2 nanotubes. As a classic approach toward nanostructured materials, the molten salt method is simple and economic,7 and has been applied to the large scale synthesis of 1D nanostructures. Surfactants can be used as templates to direct their formation.8 In this work, trigonal phase Li9V3(P2O7)3(PO4)2 nanotubes were first prepared without the use of a template and the nanotubes showed excellent cycling stability and a high rate capability as cathode materials for use in lithium-ion batteries.
To prepare the Li9V3(P2O7)3(PO4)2 nanotubes, VCl2 was added to a mixture of molten LiH2PO4 and NaNO3 salts (weight ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 580 °C under an Ar atmosphere (wt% of VCl2 in the system was ca. 8.5%). After a 10 min reaction and then cooling to room temperature the product was obtained by washing the fused material with deionized water several times to remove the nitrates. It was then air dried at 100 °C for 12 h.
2) at 580 °C under an Ar atmosphere (wt% of VCl2 in the system was ca. 8.5%). After a 10 min reaction and then cooling to room temperature the product was obtained by washing the fused material with deionized water several times to remove the nitrates. It was then air dried at 100 °C for 12 h.
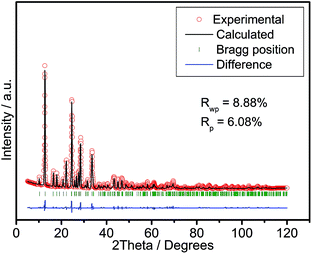
Fig. 1 shows the powder X-ray diffraction (XRD) pattern of the product, which was indexed to the trigonal phase of Li9V3(P2O7)3(PO4)2 (space group: P3c1) without any impurity phase using the Dicvol program.1a–c The lattice constants a and b are 0.9724 nm and 1.3596 nm, respectively. This was obtained from a Rietveld refinement by choosing the P3c1 space group as a model and these values are in good agreement with literature values.1a–c The small Rwp factor (8.88%) obtained further indicated that the as-prepared Li9V3(P2O7)3(PO4)2 was free of impurities.9
Scanning transmission electron microscopy (STEM) (Fig. 2a) shows that the product consists of uniform nanotubes with average diameters of 10 nm and lengths of 100 nm. The microstructure of the Li9V3(P2O7)3(PO4)2 nanotubes was further analyzed by high-resolution STEM, as shown in Fig. 2c. The lattice fringes are clearly shown in Fig. 2c. These lattice fringes indicate that the product is highly crystalline. The interplanar distance between the lattice fringes was found to be 0.491 nm, and this can be indexed to the (110) crystal planes of Li9V3(P2O7)3(PO4)2. The SAED pattern of the Li9V3(P2O7)3(PO4)2 nanotubes (Fig. 2d) indicates the polycrystalline nature of the sample. Moreover, no visible gap was detected and this implies that the nanotubes have good mechanical integrity. The highly robust nature of these nanotubes was further verified by sonicating the sample for 2 h (Fig. S1†). From the STEM images, no notable variation in morphology or collapse of the nanotube structure is evident. This excellent structural stability is required for reversible lithium storage with a prolonged cycle life. Energy dispersive X-ray (EDX) spectroscopy (Fig. S2†) showed signals for V, O and P without any impurities (such as Na or Cl), indicating that the product is very pure. The Li9V3(P2O7)3(PO4)2 nanotubes have a relatively high Brunauer–Emmett–Teller (BET) specific surface area of 57.8 m2 g−1 (Fig. S3†). The adsorption at 1382 cm−1 in the FT-IR spectrum of Li9V3(P2O7)3(PO4)2 (Fig. S4†) indicates the presence of NO32− on the surface of the nanotubes.10 In addition to NO32−, absorbed water and surface hydroxyls (bands around 3400 cm−1, 2904 cm−1, and 1640 cm−1) are also present.10 The peaks between 1100 and 400 cm−1 are attributed to P–O band vibrations.10 The bands at 515.8 and 523.6 eV in the V 2p XPS spectrum (Fig. S5†) are assigned to V 2p1/2 and V 2p3/2, respectively, indicating a V(III) state in the Li9V3(P2O7)3(PO4)2 nanotubes.11 Although NO32− species were detected in the IR spectrum, no bands for N from NO32− anions were detected by XPS (owing to its low sensitivity).
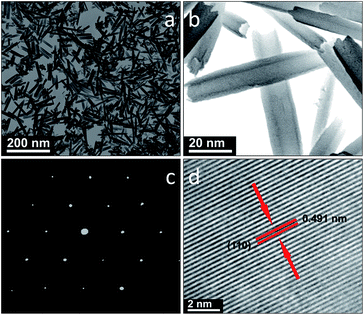 | ||
| Fig. 2 (a) Low magnification STEM image, (b) enlarged STEM image; (c) high-resolution STEM image; (d) SAED pattern of the as-prepared Li9V3(P2O7)3(PO4)2 nanotubes. | ||
The formation of the Li9V3(P2O7)3(PO4)2 nanotubes was tracked by time-dependent experiments (Fig. 3a–f). The experiments indicated that the product was mainly V2O3 (JCPDS, no.85-1411) with a small amount of Li9V3(P2O7)3(PO4)2 after a 30 s reaction (Fig. 3b). This was attributed to the thermal decomposition and oxidation of VCl2 to V2O3. Some V2O3 reacted with LiH2PO4 to form Li9V3(P2O7)3(PO4)2. With an increase in the reaction time, the amount of Li9V3(P2O7)3(PO4)2 increased and the amount of V2O3 decreased (Fig. 3c and d). After a 10 min reaction pure Li9V3(P2O7)3(PO4)2 was formed (Fig. 3e). A further increase in the reaction time did not lead to any further obvious change as determined by the corresponding XRD patterns (Fig. 3f). The reaction took place in two discrete steps: the oxidation of V2+ to V3+ (eqn (1), fast step) and Li9V3(P2O7)3(PO4)2 formation (eqn (2), decisive step). The V2+ cations were first oxidized to V2O3 with the elimination of NO2 and O2 and then V2O3 reacted with LiH2PO4 to form Li9V3(P2O7)3(PO4)2.
| 2V2+ + 4NO3− → V2O3 + 4NO2 + 1/2O2 | (1) |
| 3V2O3 + 18LiH2PO4 → 2Li9V3(P2O7)3(PO4)2 + 18H2O + P2O5 | (2) |
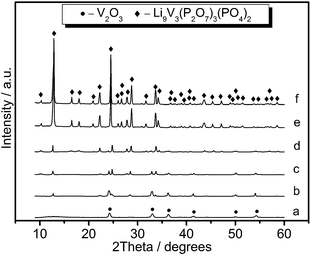 | ||
| Fig. 3 XRD patterns of the samples obtained at 580 °C for (a) 10 s, (b) 30 s, (c) 1 min, (d) 5 min, (e) 10 min and (f) 15 min. | ||
The evolution of product morphology during the reaction process was also monitored. The results indicate that a lamella-rolling mechanism is reasonable for the formation of the Li9V3(P2O7)3(PO4)2 nanotubes. As shown in Fig. 4a, V2O3 in the initial stage had a lamellar morphology. As the reaction proceeded, Li9V3(P2O7)3(PO4)2 was formed (Fig. 4b–d) and then some lamellae curling occurred leading to the formation of tube-like morphologies (Fig. 4b and c). The XRD patterns indicate the coexistence of V2O3 and Li9V3(P2O7)3(PO4)2 phases at this stage (Fig. 3b–d). At a reaction time of 10 min, all the lamellae transformed to tube-like morphology (Fig. 4d) and the XRD pattern shows that the product is pure Li9V3(P2O7)3(PO4)2 (Fig. 3e). We propose that the driving force of this transformation is the combined effects of the molten salt medium and the reaction between Li+, PO43− and V2O3. The molten salt is strongly polar and it has high surface tension. V2O3 lamellae with a large surface and high surface energy exist in a kinetically metastable state and their edges exhibit high activity. Once Li9V3(P2O7)3(PO4)2 starts to nucleate on V2O3 lamella, polar interfaces with a lower energy than the lamella are formed. To decrease the surface energy the Li9V3(P2O7)3(PO4)2 lamellae can curl and transform to tube-like morphology. The strong polarity and high tension of the molten salt medium may favor this transformation.
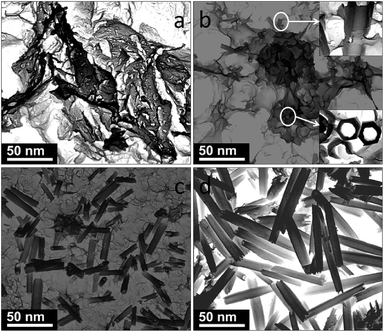 | ||
| Fig. 4 STEM images of the samples with different reaction times: (a) 30 s, (b) 1 min, (c) 3 min, and (d) 10 min. | ||
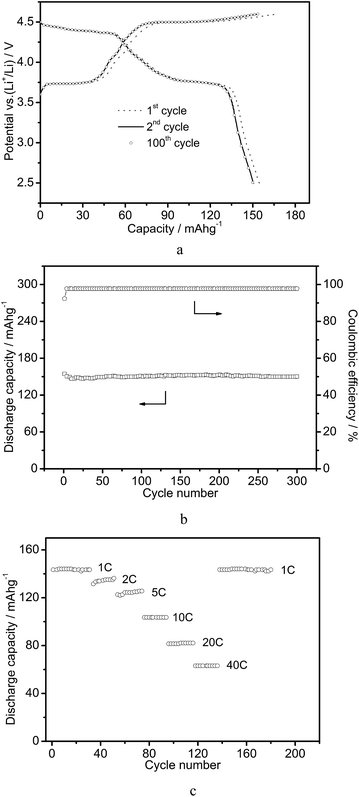
To demonstrate the advantages of these robust Li9V3(P2O7)3(PO4)2 nanotubes, we evaluated their performance as cathode materials in LIBs. Fig. 5a shows representative discharge–charge voltage profiles within a cut-off voltage window of 2.5–4.6 V versus Li+/Li. Two notable voltage plateaus are present in the discharge–charge curves at approximately 3.8 V and 4.5 V versus Li+/Li, respectively. This corresponds to a lithium insertion/de-insertion process.1 The initial charge and discharge capacities are 167.4 and 154.6 mA h g−1, respectively, leading to a high coulombic efficiency of 92.4%. Additionally, discharge and charge curves of the second and the hundredth cycles are almost identical indicating that the electrochemical process is stable during the lithium insertion/de-insertion reactions.12 Fig. 5b shows the cycling performance of the Li9V3(P2O7)3(PO4)2 nanotubes at a current density of 0.1 C (1 C = 170 mA h g−1). The capacity decays from 154.6 to 146.8 mA h g−1 over the first 10 cycles and remains very stable up to 300 cycles. The long term cycling stability of our Li9V3(P2O7)3(PO4)2 nanotubes is superior to that of other reported Li9V3(P2O7)3(PO4)2 materials.1a,b Moreover, these Li9V3(P2O7)3(PO4)2 nanotubes exhibit excellent rate capability at discharge–charge current rates ranging from 1 to 40 C, as shown in Fig. 5c. The average specific capacities are 143.2, 131.4, 122.7, 103.9, 81.3, and 63.2 mA h g−1 at current rates of 1, 2, 5, 10, 20 and 40 C, respectively. After high rate discharge–charge cycling, a specific capacity of 143.2 mA h g−1 was restored when the current density was decreased to 1 C. The rate performance of the Li9V3(P2O7)3(PO4)2 nanotubes was better than those previously reported for Li9V3(P2O7)3(PO4)2 and similar electrodes (Fig. S6†). Moreover, a study of the material after the cycling experiments revealed that the nanotube structure is perfectly retained after cycling at 5 C over 100 cycles (Fig. S7†). This indicates the excellent structural robustness of these Li9V3(P2O7)3(PO4)2 nanotubes. These results clearly demonstrate the superior lithium storage properties of Li9V3(P2O7)3(PO4)2 nanotubes in terms of long cycle life and good rate capability for the fast charging/discharging process. The outstanding electrochemical performance of the as-prepared Li9V3(P2O7)3(PO4)2 nanotubes as cathode materials for LIBs can be understood from several perspectives. First, the small size of the robust nanotubes and the relatively thin wall offer a short diffusion distance for Li+ ions. This promotes fast and reversible lithium insertion and extraction. Second, the hollow structure with high surface area provides more active surface sites and electrolyte–electrode interface compared with solid counterparts. Third, the high crystallinity of Li9V3(P2O7)3(PO4)2 nanotubes may contribute to the electrical conductivity and the crystal lattice robustness during repeated charge–discharge cycling. These features promote the electrochemical process and lead to high specific capacity especially at high rates.
Conclusions
In summary, Li9V3(P2O7)3(PO4)2 nanotubes were successfully fabricated by a simple one-pot molten salt approach. This method is facile and suitable for the low cost and large scale production of Li9V3(P2O7)3(PO4)2 nanotubes. The reaction process and the formation mechanism of the nanotubes are discussed and were found to be beneficial to the study of 1D nanostructures. Owing to their robust structure the Li9V3(P2O7)3(PO4)2 nanotubes exhibit exceptional performance with ultrastable capacity retention over 300 cycles and excellent rate capability at up to 40 C. This work is expected to be useful for the development of high-performance Li9V3(P2O7)3(PO4)2-based cathodes for lithium-ion batteries.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21376033), the Sichuan Youth Science and Technology Innovation Research Team Funding Scheme (2013TD0005), the key item of Mineral Resources Chemistry Key Laboratory of Sichuan Higher Education Institutions (KZH201103), the Cultivating programme of Middle aged backbone teachers (HG0092), the Cultivating programme for Excellent Innovation Team of Chengdu University of Technology (HY0084) and Innovative Experimental Items for College Students of Sichuan Province (SZH1106CX04).Notes and references
- (a) Q. Kuang, J. T. Xu, Y. M. Zhao, X. L. Chen and L. Q. Chen, Electrochim. Acta, 2011, 56, 2201 CrossRef CAS; (b) Q. Kuang, Z. P. Lin, Y. M. Zhao, X. L. Chen and L. Q. Chen, J. Mater. Chem., 2011, 21, 14760 RSC; (c) Q. Kuang, Y. M. Zhao and J. T. Xu, J. Phys. Chem. C, 2011, 115, 8422 CrossRef CAS; (d) J. T. Xu, Y. M. Zhao, Q. Kuang and Y. Z. Dong, Electrochim. Acta, 2011, 56, 6562 CrossRef CAS; (e) Q. Kuang and Y. M. Zhao, Electrochim. Acta, 2011, 58, 296 CrossRef CAS; (f) G. F. Gu, D. M. Tang, P. Wu, H. Y. Tian and D. G. Tong, Mater. Lett., 2013, 92, 247 CrossRef CAS; (g) Z. Y. Liang and Y. M. Zhao, Electrochim. Acta, 2013, 94, 374 CrossRef CAS; (h) X. H. Lin, Y. M. Zhao, Q. Kuang, Z. Y. Liang, D. L. Yan, X. D. Liu and Y. Z. Dong, Solid State Ionics, 2014, 259, 46 CrossRef CAS; (i) J. J. Zeng, Y. M. Zhao, Z. Y. Liang and Y. Z. Dong, J. Solid State Electrochem., 2014, 18, 561 CrossRef CAS.
- (a) G. L. Che, B. B. Lakshmi, E. R. Fisher and C. R. Martin, Nature, 1998, 393, 346 CrossRef CAS; (b) S. W. Choi, S. M. Jo, W. S. Lee and Y. R. Kim, Adv. Mater., 2003, 15, 2027 CrossRef CAS; (c) M.-S. Park, G.-X. Wang, Y.-M. Kang, D. Wexler, S.-X. Dou and H.-K. Liu, Angew. Chem., Int. Ed., 2007, 46, 750 CrossRef CAS PubMed; (d) D. G. Tong, Y. L. Li, W. Chu, P. Wu and F. L. Luo, Dalton Trans., 2011, 40, 4087 RSC; (e) J.-Y. Liao, B.-X. Lei, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, Energy Environ. Sci., 2012, 5, 5750 RSC; (f) G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. (David) Lou, Energy Environ. Sci., 2012, 5, 9453 RSC; (g) L.-N. Gao, X.-F. Wang, Z. Xie, W.-F. Song, L.-J. Wang, X. Wu, F.-Y. Qu, D. Chen and G.-Z. Shen, J. Mater. Chem. A, 2013, 1, 7167 RSC; (h) W.-Q. Wu, Y.-F. Xu, C.-Y. Su and D.-B. Kuang, Energy Environ. Sci., 2014, 7, 644 RSC; (i) Q. Lu, G. S. Hutchings, Y. Zhou, H.-L. L. Xin, H.-M. Zheng and F. Jiao, J. Mater. Chem. A, 2014, 2, 6368 RSC; (j) Y. J. He, J. F. Peng, W. Chu, Y. Z. Li and D. G. Tong, J. Mater. Chem. A, 2014, 2, 1721 RSC.
- (a) R. M. Penner, M. J. Heben, T. L. Longin and N. S. Lewis, Science, 1990, 250, 1118 CAS; (b) C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607 CrossRef CAS PubMed; (c) Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS; (d) C. Burda, X. Chen, R. Narayanan and M. A. EI-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed; (e) B. Su, Y.-C. Wu and L. Jiang, Chem. Soc. Rev., 2012, 41, 7832 RSC; (f) M.-A. Einarsrud and T. Grande, Chem. Soc. Rev., 2014, 43, 2187 RSC; (g) J. L. Xie, C. X. Guo and C. M. Li, Energy Environ. Sci., 2014, 7, 2559 RSC.
- (a) S.-H. Kang, J. B. Goodenough and L. K. Rabenberg, Chem. Mater., 2001, 13, 1758 CrossRef CAS; (b) D. H. Park, S. T. Lim, S.-J. Hwang, C.-S. Yoon, Y.-K. Sun and J.-H. Choy, Adv. Mater., 2005, 17, 2834 CrossRef CAS; (c) R. Liu and S. B. Lee, J. Am. Chem. Soc., 2008, 130, 2942 CrossRef CAS PubMed; (d) J.-Y. Gong, S.-R. Guo, H.-S. Qian, W.-H. Xu and S.-H. Yu, J. Mater. Chem., 2009, 19, 1037 RSC; (e) L.-Q. Mai, Q.-L. Wei, Q.-Y. An, X.-C. Tian, Y.-L. Zhao, X. Xu, L. Xu, L. Chang and Q.-J. Zhang, Adv. Mater., 2013, 25, 2969 CrossRef CAS PubMed; (f) F. Yang, Y. Z. Li, W. Chu, C. Li and D. G. Tong, Catal. Sci. Technol., 2014, 4, 3168 RSC; (g) F. Yang, S. Y. Fu, W. Chu, C. Li and D. G. Tong, RSC Adv., 2014, 4, 45838 RSC.
- (a) B. Gates, Y.-D. Yin and Y.-N. Xia, J. Am. Chem. Soc., 2000, 122, 12582 CrossRef CAS; (b) B. Zhang, W.-Y. Hou, X.-C. Ye, S.-Q. Fu and Y. Xie, Adv. Funct. Mater., 2007, 17, 486 CrossRef CAS; (c) H.-T. Zhu, H. Zhang, J.-K. Liang, G.-H. Rao, J.-B. Li, G.-Y. Liu, Z.-M. Du, H.-M. Fan and J. Luo, J. Phys. Chem. C, 2011, 115, 6375 CrossRef CAS; (d) B. Kim and B.-K. Park, Electron. Mater. Lett., 2012, 8, 33 CrossRef CAS; (e) T.-Y. Wei, H.-Y. Chang and C.-C. Huang, RSC Adv., 2013, 3, 13983 RSC.
- (a) S.-Y. Xie, C.-F. Wang, X.-H. Zhang, Z.-Y. Jiang, Z.-X. Xie, Z.-Q. Tian, R.-B. Huang and L.-S. Zheng, J. Mater. Chem., 2003, 13, 1447 RSC; (b) X.-M. Li, Y. Li, S.-Q. Li, W.-W. Zhou, H.-B. Chu, W. Chen, I. L. Li and Z.-K. Tang, Cryst. Growth Des., 2005, 5, 911 CrossRef CAS; (c) H. Zhang, M. Zuo, S. Tan, G.-P. Li, S.-Y. Zhang and J.-G. Hou, J. Phys. Chem. B, 2005, 109, 10653 CrossRef CAS PubMed; (d) B. Zhang, X.-C. Ye, W. Dai, W.-Y. Hou, F. Zuo and Y. Xie, Nanotechnology, 2006, 17, 385 CrossRef CAS; (e) B. Zhou, J.-R. Zhang, L. Zhao, J.-M. Zhu and J.-J. Zhu, Ultrason. Sonochem., 2006, 13, 352 CrossRef CAS PubMed; (f) B. Zhang, W.-Y. Hou, X.-C. Ye, S.-Q. Fu and Y. Xie, Adv. Funct. Mater., 2007, 17, 486 CrossRef CAS; (g) G.-W. She, W.-S. Shi, X.-H. Zhang, T.-L. Wong, Y. Cai and N. Wang, Cryst. Growth Des., 2009, 9, 663 CrossRef CAS.
- (a) M. J. Geselbracht, L. D. Noailles, L. T. Ngo, J. H. Pikul, R. I. Walton, E. S. Cowell, F. Millange and D. O'Hare, Chem. Mater., 2004, 16, 1153 CrossRef CAS; (b) X.-F. Wu, H. Bai, C. Li, G.-W. Lu and G.-Q. Shi, Chem. Commun., 2006, 1655 RSC; (c) J. Liu, W.-J. Wang, Z.-P. Guo, R. Zeng, S.-X. Dou and X.-L. Chen, Chem. Commun., 2010, 46, 3887 RSC; (d) M. V. Reddy, C. Yu, J.-H. Fan, K. P. Loh and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 4361 CrossRef CAS PubMed; (e) R.-C. Jin, L.-X. Yang, G.-H. Li and G. Chen, RSC Adv., 2014, 4, 32781 RSC; (f) Y. Ji, X.-Y. Liu, W. Liu, Y. Wang, H.-D. Zhang, M. Yang, X.-F. Wang, X.-D. Zhao and S.-H. Feng, RSC Adv., 2014, 4, 50220 RSC.
- (a) W. Wang, C. Xu, X. Wang, Y. Liu, Y. Zhan, C. Zheng, F. Song and G. Wang, J. Mater. Chem., 2002, 12, 1922 RSC; (b) C.-Y. Xu, Q. Zhang, H. Zhang, L. Zhen, J. Tang and L.-C. Qin, J. Am. Chem. Soc., 2005, 127, 11584 CrossRef CAS PubMed; (c) D. Choi, D.-H. Wang, I.-T. Bae, J. Xiao, Z.-M. Nie, W. Wang, V. V. Viswanathan, Y. J. Lee, J.-G. Zhang, G. L. Graff, Z.-G. Yang and J. Liu, Nano Lett., 2010, 10, 2799 CrossRef CAS PubMed; (d) C. Karakaya, Y. Türker and Ö. Dag, Adv. Funct. Mater., 2013, 23, 4002 CrossRef CAS; (e) Y. Ji, X.-Y. Liu, W. Liu, Y. Wang, H.-D. Zhang, M. Yang, X.-F. Wang, X.-D. Zhao and S.-H. Feng, RSC Adv., 2014, 4, 50220 RSC.
- (a) C. H. Jiang and C. Z. Yang, Analysis of materials by Ray-diffraction and scattering, Press of Higher Education, Beijing, 2010 Search PubMed; (b) J. Rodriguez-Carvajal, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 192, 55 CrossRef CAS.
- (a) X. Y. Jin, S. L. Chen and E. N. Yao, Application Guide of Infrared Spectra, Science and Technology Press of Tian Jin, Tian Jin, 1992 Search PubMed; (b) R. A. Nyquist and R. O. Kagel, Infrared Spectra of Inorganic Compounds (3800–45 cm−1), Academic press, New York, 1971 Search PubMed.
- Reference: Manual for Operator for PHI PC Windows Software Version 1.2b, Physical Electronic Division, Perkin-ElmerSoft.
- Q. X. Cha, Kinetics of Electrode, Science Press, Beijing, 2002 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13153c |
| This journal is © The Royal Society of Chemistry 2015 |