Sulfur-graphene composite with molybdenum particles for stabilizing lithium–sulfur batteries†
Zhaoling Ma,
Qiuhong Liu and
Shuangyin Wang*
State Key Laboratory of Chem/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082, P. R. China. E-mail: shuangyinwang@hnu.edu.cn
First published on 19th November 2014
Abstract
Commercial Mo particles coated with an oxide layer of MoO3 were introduced into the sulfur-graphene cathode of Li–S battery. Mo particles in the core could improve the electronic conductivity of the electrode and MoO3 layer could alleviate polysulfides diffusion, which enhances the long life cycle. Note that the discharge capacity retention was 71.8% after 100 cycles at 0.1 C in the presence of 10 wt% Mo particles.
In the field of batteries, lithium–sulfur (Li–S) batteries are the most attractive and a possible solution for the increasing energy demand. Sulfur, as a cathode material, is low-cost, rich in nature, and most importantly has five times higher theoretical energy density (1675 mA h g−1) than the present lithium ion batteries despite its low voltage window of 2.1 V.1 Li–S batteries suffer from two principal technical issues: the poor conductivity of sulfur and the severe shuttle effect of polysulfides, which seriously hinders the commercial application of Li–S batteries.2 The poor conductivity of sulfur results in the low utilization of the inner layer of the sulfur particles, which cannot fully contact and interact with the electrolyte. During the discharge, sulfur in the form of S8 is first reduced to soluble polysulfides (Li2Sn, 4 ≦ n ≦ 8), which can diffuse into the electrolyte, gather on the surface of the anodic lithium metal and directly reduced to final discharge products (Li2S2 or Li2S), thus decreasing the utilization of sulfur and specific discharge capacity during practical operation.3,4
For addressing these issues, highly conductive carbon materials with meso-/micro-pores are employed as the conductive host to suppress the shuttle effect of soluble polysulfides and to improve the utilization of sulfur cathode, the discharge capacity and cycling durability. Carbon material, especially meso-/micro-porous carbon as the host of the insulated sulfur, is a common strategy. Meso-/micro-porous carbon in the S/C composite not only has good electronic conductivity but also possesses abundant porosities, which offer capillary adsorption driving force for the soluble polysulfides and also lower their diffusion towards the lithium metal side. Surface modification of the one-dimensional carbon nanotube and two-dimensional graphene has been performed to further suppress the shuttle effect of polysulfides in Li–S batteries. The modified oxygen-containing groups, especially hydroxyl groups on the surface of carbon, have a strong absorption capacity for the soluble polysulfides through the formation of a chemical bond between hydroxyl group and sulfur element5,6 or through stronger electrostatic interactions caused by increased polarization and asymmetrical charge distribution in the S3 cluster.7 Moreover, carbon doped with nitrogen and/or boron8,9 is also reported to promote the adhesive ability of polysulfides on carbon support and the cycling stability for Li–S batteries.
Recently, more attention has been focused metals/metal oxides in order to retain the polysulfide species within the cathode. For example, Mg0.6Ni0.4O10 and Al2O3 (ref. 11) nanoparticles of about 50 nm were employed to avoid the diffusion of polysulfides from the sulfur cathode into the liquid electrolyte; however, good cycle stability is only observed for few dozens of cycles and for relatively low sulfur loadings. Nazar's group even reported that porous silica (SBA-15) as an additive mixed with S/mesoporous carbon can reversibly adsorb and desorb the intermediate polysulfides through weak bonding.12 Moreover, they also observed that α-TiO2 can interact with sulfur because of the existence of S–Ti–O.13
In this work, we selected Mo particles coated with an oxide layer of MoO3 and directly mixed them with sulfur–graphene composite at a high sulfur loading of 79.5% while preparing the slurry for the cathode of Li–S batteries. On the one hand, the high conductivity of the inner Mo metal enhances the conductivity of sulfur cathode. On the other hand, MoO3 on the outer surface of Mo particles can inhibit the shuttle effect of polysulfides by the redox reaction between Mo6+ and sulfur/polysulfides. With the good conductivity of inner Mo particles and the oxidation ability of outer MoO3, S–G-10 (the weight percentage of Mo in the composite is 10%) exhibits excellent capacity retention of 71.8% at 0.1 C for 100 cycles.
Sulfur–graphene composites were prepared using the vapor phase method by thermally treating a mixture of elemental sulfur and thermally exfoliated graphene under vacuum (for details, see the ESI†). Fig. 1a shows the scanning electron microscopy (SEM) image of the porous, thermally exfoliated and reduced graphene. The porous structure of the as-obtained graphene offers abundant porosity to accommodate sulfur with uniform distribution. After loading 79.5 wt% of sulfur, S–G has no obvious bulk of sulfur (Fig. 1b) and still remains porous, which favors electronic and ionic transport pathway. EDS mapping reveals the distribution of C and S, further confirming the uniform dispersion of elemental sulfur (Fig. 1c and d). Furthermore, the XRD pattern of S–graphene composite in Fig. S1† shows the characteristic peaks of graphene and sulfur, indicating the successful infiltration of sulfur into the graphene host.
 | ||
| Fig. 1 SEM images of graphene (a) and S/G composite (b); EDS mapping of carbon (c) and sulfur (d) of S/G composite. | ||
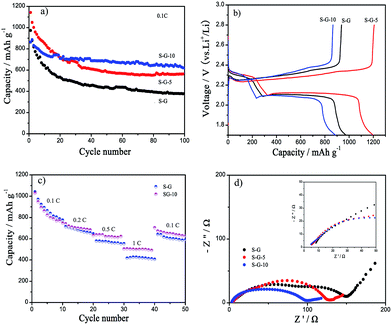
The as-prepared S–G-10 electrode was subjected to charge/discharge cycling in Li–S batteries to investigate the contribution of Mo particles to the cycling performance of Li–S batteries. For comparison, conventional S–G without Mo particles and S–G-5 with 5 wt% Mo particles were also prepared and used as the cathodes of Li–S batteries. Fig. 2a shows the life cycle of S–G, S–G-5 and S–G-10 electrodes at the current density of 0.1C. As shown in Table S1† and Fig. 2a, both S–G-5 and S–G-10 electrodes exhibit better capacity retention than S–G (71.8% for S–G-10, 49.2% for S–G-5, and 38.9% for S–G). With increase in the Mo content in the composite from 5% for S–G-5 to 10% for S–G-10, the capacity retention further improved to 71.8%. This comparison clearly demonstrated the significant role of Mo particles in enhancing the durability of the Li–S batteries in the composite. It should be noted that both S–G-5 and S–G-10 show higher capacity than S–G through almost every cycle.
Cyclic voltammetry (CV) measurements were performed to depict the effect of Mo particles on the electrochemical behavior of Li–S batteries (Fig. S2†). All CV curves have one anodic peak, corresponding to the transformation from low-order polysulfides to sulfur, and two cathodic peaks, corresponding to the reduction of sulfur in two steps. The two cathodic peaks of S–G electrode are located at lower voltages of about 1.85 V and 1.95 V, suggesting the occurrence of severe polarization. For S–G-5 and S–G-10 electrodes, cathodic peaks shift up to positive voltages and become sharper, indicating the decreased polarization and higher discharge capacity. Typical initial charge/discharge curves of S–G, S–G-5, S–G-10 electrodes in Fig. 2b display one charge plateau at about 2.4 V and two discharge plateaus at about 2.3 V and 2.1 V, which are assigned to quick charge process and two-step discharge, respectively. Fig. 2c shows the rate performance of S–G and S–G-10 electrodes. At the current density of 0.1 C, S–G electrode has a slightly higher discharge capacity than S–G-10 electrode, but at 0.2 C, 0.5 C and 1 C, S–G-10 electrode displays higher discharge capacities than S–G electrode. Furthermore, with continuous cycling, S–G-10 has a better cycle stability. Even at 0.1 C, S–G-10 electrode retains a higher discharge capacity than S–G electrode, indicating that Mo particles in the S–G-10 composite can effectively promote the cycling durability of Li–S batteries. The electrochemical impedance spectroscopy (EIS) was also performed to study the electrochemical behavior of S–G, S–G-5, and S–G-10 electrodes, as shown in Fig. 2d. S–G-10 electrode has the lowest Rs (total ohmic resistance), as shown in the inset, due to the high conductivity of Mo metal in the composite. Nyquist plots of S–G, S–G-5 and S–G-10 electrodes show two semicircles at high and middle frequency regions, and an oblique line at low frequency regions. The two semicircles present the SEI film resistance formed between anode/cathode and electrolyte as well as the charge-transfer resistance.14–16 Note that with the addition of Mo particles, both SEI film resistance and charge-transfer resistance become smaller and further incorporate into one semicircle, suggesting the decreased polarization of sulfur cathode.
In order to understand the role of Mo particles in S–G-10 composites in enhancing the cycling durability of Li–S batteries, a series of physical characterizations were performed. The transmission electron microscopy (TEM) image in Fig. 2a displays the typical graphene structure of the thermally exfoliated and reduced graphene. TEM image of S–graphene composite in Fig. 2b shows that sulfur is deposited on the surface of graphene, which increases the combination with graphene support and enhances sulfur utilization efficiency. With the addition of 10 wt% commercial Mo particles in the S–G composite, the S–G-10 sample in Fig. 2c shows that Mo particles (dark part) were dispersed in the sulfur–graphene composites. Sulfur was also observed in S–G-10 in Fig. 2c, as indicated with arrows. The as-observed particle size of Mo particles in the S–G-10 sample is consistent with that observed for pristine Mo particles in Fig. S3,† which include nanometer- and micrometer-sized Mo particles. In order to investigate the detailed structure of the Mo particle in S–G-10, TEM image with a higher resolution was obtained, which is shown in Fig. 2d. Interestingly, a core–shell structure was observed. The distinct lattice stripes of Mo particles core shown in the magnified TEM image (Fig. 2d) demonstrate the metallic characteristic of Mo particles; however, the thin outer layer is amorphous, which was considered to be MoO3, which was confirmed by X-ray spectroscopy (XPS) below. It is a general phenomenon for commercial metal particles to form a thin metal oxide layer, due to oxidation when exposed to air. Using the as-prepared S–G-10 with commercial Mo particles embedded in the S–G composite as the cathode materials of Li–S batteries, it is expected that the Mo particles coated with a thin layer of Mo oxide might contribute to the performance of Li–S batteries. As-assembled Li–S batteries with S–G-10 as the cathode and Li metal foil as the anode were subjected to cycling at a relatively low rate of 0.1 C to investigate the structural change of Mo particles in S–G-10. After 100 cycles of charge/discharge, the electrode (denoted as SG-10) was obtained by disassembling the Li–S cell and rinsing the cathode several times using the mixture of DME/DOL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After 100 cycles of charge/discharge at 0.1 C, the thin outer oxide layer of Mo particle with amorphous characteristic in SG-10 electrode becomes rough and thick (Fig. 2e), which could be a new product produced during the charge/discharge process, which was confirmed by subsequent XPS characterizations.
1, v/v). After 100 cycles of charge/discharge at 0.1 C, the thin outer oxide layer of Mo particle with amorphous characteristic in SG-10 electrode becomes rough and thick (Fig. 2e), which could be a new product produced during the charge/discharge process, which was confirmed by subsequent XPS characterizations.
To further elucidate the change of Mo particles after cycling, XPS analysis was used, investigating the chemical environment of the target element. Fig. 4 shows the fine-scanned Mo 3d peak of commercial Mo powder and SG-10 after 100 cycles of charge/discharge cycling in Li–S batteries. Mo3d spectrum of SG-10 in Fig. 4 consists of four peaks, whose positions are different from those of Mo powder. Mo 3d peaks at 228.1 eV for pristine Mo powder and 228.2 eV for Mo particles in SG-10 are attributed to Mo0. The peaks at 231.8 eV and 235.0 eV for Mo powder correspond to MoO3,17–19 revealing the oxidation of the outer surface of Mo particles. Combined with the observation of TEM in Fig. 3d, this clearly shows that a MoO3 layer on the outer surface of the commercial Mo particles was formed, probably due to the oxidation resulting from a long exposure to air. Interestingly, after 100 cycles of charge/discharge, new peaks at 232.8 and 236.2 eV were observed for Mo in SG-10 electrode, which can be assigned to Mo 3d3/2 and Mo 3d5/2 spin–orbit of MoO2.20,21 This indicates that the charge/discharge process of Li–S batteries results in the transformation of Mo oxide layer on Mo particles from MoO3 to MoO2, which confirms that the redox reaction occurred between MoO3 and reducing polysulfides.22 In addition, like other oxides such as TiO2 or even SiO2,12,13 the converted MoO2 might also be able to reduce the shuttle effect of Li–S battery. It is believed that the redox reaction between MoO3 and polysulfide could facilitate the hosting of polysulfide in the cathode and suppress the shuttle effect, which could significantly stabilize Li–S batteries with improved long-term cycle life.
 | ||
| Fig. 3 TEM images of graphene (a) and S/G composite (b); fresh S–G-10 (c and d) and S–G-10 electrode after cycling in Li–S batteries at 0.1C for 100 cycles (e and f). | ||
In summary, commercial Mo particles coated with an oxide layer of Mo oxide were introduced to improve the conductivity of sulfur cathode and cycle life of Li–S batteries. S–G-10 electrode with a high sulfur load of 79.5 wt% in S/G composite exhibits a high capacity retention of 71.8%, which is twice that of S–G electrode without addition of Mo particles. Our physical characterization demonstrated that the excellent electrochemical performance was due to the redox reaction between reducing polysulfides and oxidative MoO3, which significantly decreases the shuttle effect. This current finding presents a general way to enhance the cycling durability of Li–S batteries.
Acknowledgements
This work was supported by the National Natural of Science Foundation of China (Grant no. 51402100), the Youth 1000 Talent Program of China, and the Inter-discipline Research Program of Hunan University.Notes and references
- Y. S. Su and A. Manthiram, Nat. Commun., 2012, 3, 1166 CrossRef PubMed.
- Z. Wang, Y. Dong, H. Li, Z. Zhao, H. Bin Wu, C. Hao, S. Liu, J. Qiu and X. W. Lou, Nat. Commun., 2014, 5, 5002 CrossRef CAS PubMed.
- S.-E. Cheon, K.-S. Ko, J.-H. Cho, S.-W. Kim, E.-Y. Chin and H.-T. Kim, J. Electrochem. Soc., 2003, 150, A800 CrossRef CAS PubMed.
- P. Zhu, J. Song, D. Lv, D. Wang, C. Jaye, D. A. Fischer, T. Wu and Y. Chen, J. Phys. Chem. C, 2014, 118, 7765–7771 CAS.
- S. Evers and L. F. Nazar, Chem. Commun., 2012, 48, 1233–1235 RSC.
- C. X. Zu and A. Manthiram, Adv. Energy Mater., 2013, 3, 1008–1012 CrossRef CAS.
- G. Zhou, L. C. Yin, D. W. Wang, L. Li, S. Pei, I. R. Gentle, F. Li and H. M. Cheng, ACS Nano, 2013, 7, 5367–5375 CrossRef CAS PubMed.
- C. P. Yang, Y. X. Yin, H. Ye, K. C. Jiang, J. Zhang and Y. G. Guo, ACS Appl. Mater. Interfaces, 2014, 6, 8789–8795 CAS.
- J. X. Song, T. Xu, M. L. Gordin, P. Y. Zhu, D. P. Lv, Y. B. Jiang, Y. S. Chen, Y. H. Duan and D. H. Wang, Adv. Funct. Mater., 2014, 24, 1243–1250 CrossRef CAS.
- M. S. Song, S. C. Han, H. S. Kim, J. H. Kim, K. T. Kim, Y. M. Kang, H. J. Ahn, S. X. Dou and J. Y. Lee, J. Electrochem. Soc., 2004, 151, A791–A795 CrossRef CAS PubMed.
- Y. J. Choi, B. S. Jung, D. J. Lee, J. H. Jeong, K. W. Kim, H. J. Ahn, K. K. Cho and H. B. Gu, Phys. Scr., T, 2007, 129, 62–65 CrossRef.
- X. Ji, S. Evers, R. Black and L. F. Nazar, Nat. Commun., 2011, 2, 325 CrossRef PubMed.
- S. Evers, T. Yim and L. F. Nazar, J. Phys. Chem. C, 2012, 116, 19653–19658 CAS.
- X. Gao, J. Li, D. Guan and C. Yuan, ACS Appl. Mater. Interfaces, 2014, 6, 4154–4159 CAS.
- W. Ahn, K. B. Kim, K. N. Jung, K. H. Shin and C. S. Jin, J. Power Sources, 2012, 202, 394–399 CrossRef CAS PubMed.
- E. J. Peled, Electrochem. Soc., 1979, 126, 2047–2051 CrossRef CAS PubMed.
- W. Gruenert, A. Y. Stakheev, R. Feldhaus, K. Anders, E. S. Shpiro and K. M. Minachev, J. Phys. Chem., 1991, 95, 1323–1328 CrossRef CAS.
- J. Muijsers, J. Catal., 1995, 157, 698–705 CrossRef CAS.
- J. Swiatowska-Mrowiecka, S. de Diesbach, V. Maurice, S. Zanna, L. Klein, E. Briand, I. Vickridge and P. Marcus, J. Phys. Chem. C, 2008, 112, 11050–11058 CAS.
- J. G. Choi and L. T. Thompson, Appl. Surf. Sci., 1996, 93, 143–149 CrossRef CAS.
- Y. Sun, X. Hu, J. C. Yu, Q. Li, W. Luo, L. Yuan, W. Zhang and Y. Huang, Energy Environ. Sci., 2011, 4, 2870–2877 CAS.
- P. A. Spevack and N. S. McIntyre, J. Phys. Chem., 1993, 97, 11031–11036 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13123a |
| This journal is © The Royal Society of Chemistry 2015 |