One-pot synthesis of alkyl pyrrole-2-carboxylates starting from β-nitroacrylates and primary amines†
Alessandro Palmieri*,
Serena Gabrielli,
Marco Parlapiano and
Roberto Ballini*
Green Chemistry Group, School of Science and Technology, Chemistry Division, University of Camerino, via S. Agostino 1, 62032 Camerino, MC, Italy. E-mail: alessandro.palmieri@unicam.it; roberto.ballini@unicam.it; Fax: +39 0737 402297
First published on 9th December 2014
Abstract
Herein, we present a new, efficient, one-pot synthesis of pyrrole-2-carboxylate derivatives starting from ketal-functionalized β-nitroacrylates in combination with primary amines under acidic heterogeneous conditions.
Pyrroles constitute one of the most important classes of nitrogen-containing heterocycles and, due to their importance, several procedures for synthesizing poly-functionalized pyrroles were reported.1 In this context, pyrrole-2-carboxylate derivatives have been largely investigated for their activities,2 and exploited as strategic starting materials, or key intermediates, for the preparation of biological active molecules, such as pyrrolnitrin and porphyrins.3
Given their great importance, over the years several methodologies have been proposed in the literature for the preparation of these molecules. The first one was the pioneering Kleinspehn's method, which involves the use of diethyl α-oximinomalonate in combination with 1,3-diketones under reductive conditions,4 then, analogous procedures based on the use of α-oximinomalonate or its amino reduced form were successively proposed.5 Additional synthetic strategies were developed by Barluenga from azabutadiene,6 and Gupton from 2-substituted vinamidinium or 3-aryl-3-chloropropeniminium salts.7 Further useful syntheses were reported by Tashiro, from 1,3-diketones,8 and Guillard from β-arylacroleins.9
Although these methodologies lead to the preparation of pyrrole-2-carboxylates in efficient ways, they present important limitations such as, the need of high temperature (80–140 °C) and inert atmosphere, the use of dangerous reactants (e.g. NaH) and unsustainable solvents (e.g. DMF, pyridine, AcOH).10 Furthermore, all the reported procedures involve an articulate work-up, with evident further disadvantages from ecological point of view.
Nowadays, the sustainability of a chemical process is one of the main aspects that must be considered, and the implementation of new green methodologies is of dramatic importance.11 In this sense, following our on-going research project concerning the development of new low impacting procedures,12 we focused our attention to ketal-functionalized β-nitroacrylates type 1, an emerging class of molecules that we have recently used in our laboratory as precursor of the indole system.13 In fact, the structure 1 seems to be ideal for the ex-novo ring construction and, herein, we report a new application of 1 in combination with primary amines 2 to synthesize the title compounds 6 (Scheme 1).
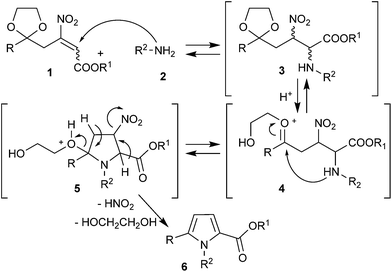
Our approach consists in a one-pot process (Scheme 2), which involves (i) an initial Michael addition of the primary ammine 2 to β-nitroacrylate 1,14 with the formation of the intermediate 3, (ii) the in situ acidic treatment of 3 giving the opening of 1,3-dioxolane ring (4), with the successive cyclization-aromatization of the former β-nitroacrylate moiety (5) and formation of pyrrole 6.
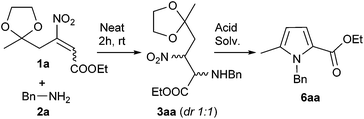
In order to find the best reaction conditions, we investigated the reaction between ethyl 4-(2-methyl-1,3-dioxolan-2-yl)-3-nitrobut-2-enoate 1a (R = Me, R1 = Et) and benzylamine 2a.
Thanks to the great reactivity of β-nitroacrylates, the conjugate addition of 2a to 1a allows the Michael adduct 3aa in quantitative yield, over 2 hours, under promoter free and solvent free conditions. On the other hands, with the aim to maximize the reaction efficiency of the cleavage–cyclization–aromatization domino process, a variety of acidic species and solvents were screened (Table 1).
| Acid (g mmol−1) | Solvent | Temp (°C) | Yielda (%) of 6aa (h) |
|---|---|---|---|
| a Yield of pure isolated product. | |||
| p-TsOH·H2O (0.19) | 2-MeTHF | 40 | 32 (6) |
| p-TsOH·H2O (0.19) | 2-MeTHF | 60 | 45 (6) |
| Amberlyst 15 (0.4) | 2-MeTHF | 40 | 39 (6) |
| Amberlyst 15 (0.4) | 2-MeTHF | 60 | 66 (3) |
| Amberlyst 15 (0.4) | 2-MeTHF | 75 | 67 (3) |
| Amberlyst 15 (0.6) | 2-MeTHF | 60 | 62 (3) |
| Amberlyst 15 (0.2) | 2-MeTHF | 60 | 68 (3) |
| Amberlyst 15 (0.1) | 2-MeTHF | 60 | 22 (3) |
| Amberlyst 15 (0.2) | CPME | 60 | 55 (3) |
| Amberlyst 15 (0.2) | EtOAc | 60 | 48 (3) |
| Amberlyst 15 (0.2) | DCM | 60 | 42 (3) |
| Acidic Al2O3 (0.2) | 2-MeTHF | 60 | — |
| Montm. K10 (0.2) | 2-MeTHF | 60 | — |
| H2SO4/SiO2 (0.2) | 2-MeTHF | 60 | 36 (3) |
| Zeolite HSZ320 (0.2) | 2-MeTHF | 60 | 11 (3) |
As reported in the Table 1, the best result for pyrrole 6aa (overall yield = 68%) was obtained over 3 hours, using Amberlyst 15 (200 mg mmol−1) in 2-MeTHF, as green solvent,15 at 60 °C.
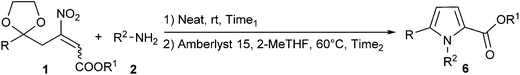
Then, we tested the generality of our protocol to a plethora of β-nitroacrylates 1 and amines 2 (Table 2). In all cases, pyrroles 6 were isolated in good overall yields (53–75%) with both aliphatic and aromatic amines, independently from the nature of substituents present on β-nitroacrylates.
| R | R1 | R2 | Time1 (h) | Time2 (h) | Yielda (%) of 6 | |||
|---|---|---|---|---|---|---|---|---|
| a Yield of pure isolated product.b The first step was performed in presence of 300 μL mmol−1 of 2-MeTHF. | ||||||||
| 1a | Me | Et | 2a | Bn | 2 | 3 | 6aa | 68 |
| 1a | Me | Et | 2b | CH3(CH2)4 | 2 | 3 | 6ab | 72 |
| 1a | Me | Et | 2c | CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2 CCH2 |
2 | 3 | 6ac | 68 |
| 1b | CH3(CH2)7 | Et | 2a | Bn | 2 | 3 | 6ba | 70 |
| 1c | p-Tol | Et | 2d | 4-MeOC6H4 | 3b | 20 | 6cd | 60 |
| 1c | p-Tol | Et | 2e | CH2 = CHCH2 | 2 | 3 | 6ce | 70 |
| 1d | Me | i-Pr | 2f | Ph | 3 | 16 | 6df | 75 |
| 1e | CH3(CH2)5 | i-Bu | 2g | i-Bu | 2 | 3 | 6eg | 73 |
| 1f | H | i-Bu | 2h | i-Pr | 2 | 15 | 6fh | 64 |
| 1f | H | i-Bu | 2f | Ph | 3 | 16 | 6ff | 63 |
| 1g | Me | Me | 2c | CH![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2 CCH2 |
2 | 3 | 6gc | 53 |
| 1g | Me | Me | 2i | 2-BnOC6H4 | 7 | 7 | 6gi | 63 |
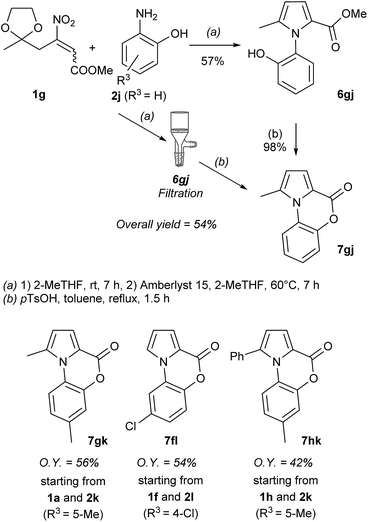
Moreover, by the appropriate selection of the amines 2, even a variety of protecting groups at N-position (benzyl: 6aa and 6ba, allyl: 6ce and PMP: 6cd), and reactive functionalities such as double (6ce) and triple (6ac and 6gc) bonds were introduced. Successively, we applied our method to synthesize pyrrolebenzoxoazinone derivatives 7 (Fig. 1), a valuable class of biologically active molecules,16 starting from 1 and aminophenols (2k–l).
As reported in the Scheme 3, the synthesis was tested studying the reaction of 1g with 2j. Applying our reaction conditions the reaction gives the pyrrole 6gj (57% yield after purification), which in turn, was cyclized into the target compound 7gj, in quantitative yield, by treatment with p-toluensulfonic acid under refluxing toluene. Alternatively, the crude intermediate 6gj can be directly converted into 7gj (54% overall yield) avoiding the purification of the intermediate and minimizing the waste production.
The synthetic process was then applied to prepare the additional derivatives 7gk, 7fl, and 7hk.
Finally, in order to automate the process, we explored the reactivity of the starting materials 1a and 2b under flow chemical conditions (Scheme 4). A preliminary screening was carried out with the aim to optimize the reaction conditions in terms of concentration, residence time and stoichiometry. The best result was achieved using 0.2 M solution of 1a and 2b in 2-MeTHF, a flow rate of 0.05 ml min−1 for each pumps, a coil reactor R1 (PTFE, i.d. = 0.5 mm) having and internal volume of 10 mL (residence time, 100 minutes), an Omnifit column reactor R2 heated at 60 °C and packed with Amberlyst 15 (1.5 g mmol−1), and a back pressure regulator (BPR) set at ∼2 atm.
Working under these reaction conditions, the pyrrole 6ab was synthesized in 70% of yield (vs. 72% in batch). The same conditions were extended to substrate 1a and 2c for synthesizing 6ac, which was isolated in 71% of yield (vs. 68% in batch). In particular the flow chemical synthesis of 6ac is of valuable interest, since it could be potentially submitted to further clickable manipulations.17
Conclusions
In conclusion, by our methodology it has been possible to synthesize several pyrroles introducing a variety of protecting group at N-position, as well as an assortment of both aliphatic and aromatics substituents at C-5. Furthermore, we demonstrated the applicability of our synthetic approach preparing benzo[b]pyrrolo[1,2-d][1,4]oxazin-4-one derivatives, and we extended our methodology to flow chemical conditions demonstrating the easy process-automation.Finally, by the choices of 2-MeTHF as solvent, and the use of Amberlyst A15 as solid acid, we could avoid any complicate aqueous work-up, saving resources and energy with evident advantages from sustainable point of view.
Acknowledgements
The authors thank the University of Camerino and MIUR-Italy (FIRB National Project “Metodologie di nuova generazione nella formazione di legami carbonio-carbonio e carbonio-eteroatomo in condizioni eco-sostenibili”) for financial support.Notes and references
- (a) R. Martín, C. H. Larsen, A. Cuenca and S. L. Buchwald, Org. Lett., 2007, 9, 3379 CrossRef PubMed; (b) N. Azizi, A. Khajeh-Amiri, H. Ghafuri, M. Bolourtchian and M. R. Saidi, Synlett, 2009, 2245 CrossRef CAS PubMed; (c) T. Maehara, R. Kanno, S. Yokoshima and T. Fukuyama, Org. Lett., 2012, 14, 1946 CrossRef CAS PubMed; (d) B. Gabriele, L. Veltri, R. Mancuso, G. Salerno, S. Maggi and B. M. Aresta, J. Org. Chem., 2012, 77, 4005 CrossRef CAS PubMed; (e) R. J. Wakeham, J. E. Taylor, S. D. Bull, J. A. Morris and J. M. J. Williams, Org. Lett., 2013, 15, 702 CrossRef CAS PubMed; (f) M. Zahng, X. Fang, H. Neumann and M. Beller, J. Am. Chem. Soc., 2013, 135, 11384 CrossRef PubMed; (g) R. Ballini, S. Gabrielli, A. Palmieri and M. Petrini, RSC Adv., 2014, 4, 43258 RSC.
- (a) M. Birouk, S. Harraga, J. Panouse-Perrini, J. F. Robert, M. Damelincourt, F. Theobald, R. Mercier and J. J. Panouse, Eur. J. Med. Chem., 1991, 26, 91 CrossRef CAS; (b) X. Teng, H. Keys, J. Yuan, A. Degterev and G. D. Cuny, Bioorg. Med. Chem. Lett., 2008, 18, 3219 CrossRef CAS PubMed; (c) F. Micheli, R. Di Fabio, A. Giacometti, A. Roth, E. Moro, G. Merlo, A. Paio, E. Merlo-Pich, S. Tomelleri, F. Tonelli, P. Zarantonello, L. Zonzini and A. M. Capelli, Bioorg. Med. Chem. Lett., 2010, 20, 7308 CrossRef CAS PubMed; (d) M. Urbano, M. Guerrero, J. Zhao, S. Velaparthi, M.-T. Schaeffer, S. Brown, H. Rosen and E. Roberts, Bioorg. Med. Chem. Lett., 2011, 21, 5470 CrossRef CAS PubMed.
- (a) J. B. Paine III, in The Porphyrins, ed. D. Dolphin, Academic Press, New York, 1978, vol. 1, p. 101 Search PubMed; (b) K. Sakata, K. Aoki, C.-F. Sakurai, S. Tamura and S. Murakoshi, Agric. Biol. Chem., 1978, 42, 457 CrossRef CAS.
- G. G. Kleinspehn, J. Am. Chem. Soc., 1955, 15, 1546 CrossRef.
- (a) J. B. Pain III and D. Dolphin, J. Org. Chem., 1985, 50, 5598 CrossRef; (b) J. B. Pain III, J. R. Brough, K. K. Buller and E. E. Erikson, J. Org. Chem., 1987, 52, 3986 CrossRef.
- J. Barluenga, V. Rubio and V. Gotor, J. Org. Chem., 1982, 47, 1696 CrossRef CAS.
- (a) G. T. Gupton, D. A. Krolikowski, R. H. Yu and S. W. Riesinger, J. Org. Chem., 1990, 55, 4735 CrossRef; (b) G. T. Gupton, D. A. Krolikowski, R. H. Yu and P. Vu, J. Org. Chem., 1992, 57, 5480 CrossRef.
- S. Mataka, K. Takahashi, Y. Tsuda and M. Tashiro, Synthesis, 1982, 157 CrossRef CAS.
- J. P. Boukou-Poba, M. Farnier and R. Guilard, Tetrahedron Lett., 1979, 20, 1717 CrossRef.
- (a) C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927 CAS; (b) K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31 RSC; (c) P. J. Dunn, Chem. Soc. Rev., 2012, 41, 1452 RSC.
- (a) P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed; (b) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed; (c) I. T. Horváth and P. T. Anastas, Chem. Rev., 2007, 107, 2169 CrossRef PubMed; (d) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC.
- (a) R. Ballini, D. Fiorini, M. V. Gil and A. Palmieri, Green Chem., 2003, 5, 475 RSC; (b) R. Ballini, D. Fiorini, M. V. Gil and A. Palmieri, Tetrahedron, 2004, 60, 2799 CrossRef CAS PubMed; (c) R. Ballini, G. Bosica, D. Fiorini and A. Palmieri, Green Chem., 2005, 7, 825 RSC; (d) R. Ballini, L. Barboni, D. Fiorini, G. Giarlo and A. Palmieri, Green Chem., 2005, 7, 828 RSC; (e) R. Ballini, L. Barboni, F. Fringuelli, A. Palmieri, F. Pizzo and L. Vaccaro, Green Chem., 2007, 9, 823 RSC; (f) R. Ballini, G. Bosica, A. Palmieri, F. Pizzo and L. Vaccaro, Green Chem., 2008, 10, 541 RSC; (g) L. Barboni, S. Gabrielli, A. Palmieri, C. Femoni and R. Ballini, Chem.–Eur. J., 2009, 15, 7867 CrossRef CAS PubMed; (h) S. Gabrielli, A. Palmieri, A. Perosa, M. Selva and R. Ballini, Green Chem., 2011, 13, 2026 RSC; (i) A. Palmieri, S. Gabrielli, C. Cimarelli and R. Ballini, Green Chem., 2011, 13, 3333 RSC; (j) A. Palmieri, S. Gabrielli and R. Ballini, Green Chem., 2013, 15, 2344 RSC.
- A. Palmieri, S. Gabrielli, D. Lanari, L. Vaccaro and R. Ballini, Adv. Synth. Catal., 2011, 353, 1425 CrossRef CAS.
- For example concerning the addition of amines to β-nitroacrylates see: (a) R. Ballini, N. A. Bazán, G. Bosica and A. Palmieri, Tetrahedron Lett., 2008, 49, 3865 CrossRef CAS PubMed; (b) A. Palmieri, S. Gabrielli, R. Maggi and R. Ballini, Synlett, 2014, 25, 128 CrossRef CAS PubMed.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369 CrossRef CAS PubMed.
- (a) G. W. H. Cheeseman, M. Rafiq, P. D. Roy, C. J. Turner and G. V. Boyd, J. Chem. Soc. C, 1971, 2018 RSC; (b) V. A. Vaillard, R. A. Rossi and S. E. Martín, Org. Biomol. Chem., 2011, 9, 4927 RSC; (c) T. Sanaeishoar, S. Adibi-Sedeh and S. Karimian, Comb. Chem. High Throughput Screening, 2014, 17, 157 CrossRef CAS; (d) R. T. Naganaboina, A. Nayak and R. K. Peddinti, Org. Biomol. Chem., 2014, 12, 3366 RSC; (e) L. Moradi, M. Piltan and H. Rostami, Chin. Chem. Lett., 2014, 25, 123 CrossRef CAS PubMed.
- Dr. Reddy's Laboratories Limited and Dr. Reddy's Laboratories, Inc., WO2008/143649 (A2), 2008.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13094d |
| This journal is © The Royal Society of Chemistry 2015 |