Mesoporous materials: versatile supports in heterogeneous catalysis for liquid phase catalytic transformations
Nabanita Pal
*a and
Asim Bhaumik
*b
aSurface Physics Division, Saha Institute of Nuclear Physics, 1/AF Bidhannagar, Kolkata-700 064, India. E-mail: nabanita.pal@saha.ac.in; pal_nabamsc@yahoo.co.in
bDepartment of Materials Science, Indian Association for the Cultivation of Science, Jadavpur, Kolkata-700 032, India. E-mail: msab@iacs.res.in
First published on 9th February 2015
Abstract
Due to their unprecedented intrinsic structural features, like tunable pore diameter of nanoscale dimensions, huge BET surface areas and good flexibility to recognize/accommodate various functional groups and metals onto the surface, an inevitable linkage of nanoporous materials with catalysis has been built-up over the past few decades. As a result of which, a huge number of communications and articles dealing with these materials with nanoscale porosity have come to light. In this review, our objective is to provide a comprehensive overview of mesoporous solids, the most remarkable member of the nanoporous family of materials, the general strategy for their syntheses and application of functionalized porous materials in liquid phase catalytic reactions. In the latter part, the role of catalytic centres in various organic transformations over these functionalized mesoporous materials and their economical, environmental and industrial aspects are described in detail.
1. Introduction
Today, worldwide over 60% of industrially important chemical products are produced by different chemical process and the majority of these processes are accelerated by using suitable catalysts.1 Wilhelm Ostwald rightly said that “there is probably no chemical reaction which cannot be influenced catalytically”.2 Catalysts play a crucial role in the manufacturing process for the synthesis of fine chemicals, pharmaceuticals, commodity chemicals, energy resources, fuels, biofuels and so on. The extraordinary advantage of heterogeneous catalysis over homogeneous catalysis is the separation, recoverability and recycling capability, which leads to compliance to green synthetic pathways involving minimum environmental pollution.Porous nanostructured materials with a huge BET surface area are suitable for catalytic reactions and in most cases the nature of the catalytically active components (e.g. metals, organic functional groups etc.) is most crucial in heterogeneous catalysts. Hence, researchers are very keen to design hierarchical porous nanomaterials bearing catalytically active centers at their surface. A number of reviews by Pérez-Ramírez et al.,3 Pal et al.,4 Lopez-Orozco et al.5 and others on the synthesis and applications of porous solids have been reported in recent times. The introduction of microporous solids, particularly zeolites in industrial manufacturing processes, has brought an enormous economical and environmental revolution.6 These microporous materials play a key role in shape-selective catalysis on the industrial scale.7 However, their small pore opening has motivated researchers to focus their attention on designing materials with pores of still larger dimensions in the nanoscale region. This ultimately leads to the discovery of ordered mesoporous silicas through supramolecular templating pathways.8 Later, other mesoporous supports like mesoporous oxides, phosphates, carbons, etc. were invented and they play a significant role in industrial manufacturing processes for fine chemicals. Liquid phase catalytic transformations in heterogeneous media over mesoporous solids are more favorable, and they lead to minimum diffusion as compared to other materials belonging to the porous categories.
A number of comprehensive reviews in the literature related to the catalytic application of mesoporous solids by Corma,9 Taguchi et al.,10 Perego et al.11 and Martín-Aranda et al.12 have arisen, which mainly discussed catalysis over ordered mesoporous silica based materials. The other mesoporous materials like oxides, phosphates, carbons, polymers and organic–inorganic hybrid periodic mesoporous organosilicas,13 which are of parallel importance as far as industrial catalysis is concerned, have had less emphasis in these reviews. The present review illustrates an overall picture of heterogeneous catalytic reactions mediated by a wide range of mesoporous materials invented so far and their environmental aspects for clean chemical synthesis.
2. What are mesoporous materials?
Traditional porous materials, the more commonly used term being ‘nanoporous’ materials, are defined as a continuous and solid network material filled through voids of ‘nanoscale pores’ of the order of say 100 nm or smaller. The International Union of Pure and Applied Chemistry (IUPAC) have classified nanoporous solids into three categories according to the pore size they possess: microporous with a pore diameter of less than 2 nm; mesoporous having a pore diameter in the range of 2–50 nm; and macroporous with a greater than 50 nm pore size.14 There are some recent reports on catalytic activity over macroporous materials,15,16 but owing to the low surface area of nanoporous materials of this category they are not in such high demand for liquid phase heterogeneous catalysis. Also many microporous zeolites, aluminophosphates, organic–inorganic hybrid phosphonates, and metal organic frameworks (MOFs) are quite useful for environmentally benign green catalytic reactions,17,18 but those are out of the scope of this review, where our discussion will be focused on mesoporous solids and their potential in heterogeneous catalysis.The history of mesoporous molecular sieves (where, ‘meso’ the Greek prefix, meaning “in between”, adopted by IUPAC for dimensions of pores typically between 2 and 50 nm) begins in the last decades of the twentieth century when materials like MCM (Mobil Composition of Matter)-type materials8 were successfully synthesized by Mobil scientists. The discovery of the M41S family of ordered mesoporous materials with a pore dimension of 2–10 nm, high surface area (∼1000 m2 g−1) and large pore volume (∼1.0 cm3 g−1), by using quaternary alkylammonium surfactants (e.g. cetyltrimethylammonium bromide, CH3(CH2)15N(CH3)3+Br−) as a template, can be considered one of the most important milestones in the history of the porous materials world, resulting in huge expectations for their application as heterogeneous catalysts.19,20
Among the ordered mesoporous materials discovered initially, mesoporous silicas, e.g. MCM-41, MCM-48, MCM-50, FSM-16,21 SBA-15 (ref. 22) etc., are well-known. Though there is some difference in the synthesis conditions and structural properties of these silicas, the basic strategy of synthesis is similar in all cases, and is based on the supramolecular self-assembly of the surfactants (or templates).23 Rather than a small organic molecule as the single molecule template (SDA), long chain cationic surfactant molecules, like CTAB (cetyltrimethylammonium bromide), CPC (cetylpyridinium chloride) etc. or anionic surfactants like SDS (sodium dodecyl sulphate) are used during the synthesis of highly ordered silica based materials.8 In solution, co-operative self-assembly of surfactant molecules takes place at concentrations higher than the CMC when a spherical or rod shaped geometry is obtained around which hydrolyzed inorganic silica precursors (say, tetraethyl orthosilicate or TEOS) arrange themselves in order to form organic–inorganic metal-template composites in a highly basic medium. Through ageing for a particular time further condensation occurs and the composites take on an ordered arrangement such as cubic or hexagonal, etc. After removal of the surfactants from the composite materials through calcination or solvent-extraction we can obtain the final mesoporous solids free from organic templates. A detailed synthesis strategy for mesoporous silica and other mesoporous solids using a surfactant-templated route based on the selection of different types of surfactants has been extended in the review published recently.4 A typical synthesis scheme of highly ordered 2D hexagonal pure mesoporous silica MCM-41 mediated by a surfactant is shown in Fig. 1. Depending upon the type of surfactant used, the surfactant–silica ratio and the pH of the solution, the structure and mesopore size of the silica can be varied from hexagonal (e.g. MCM-41), to cubic (e.g. MCM-48), to lamellar (e.g. MCM-50). Mesoporous SBA-15 type materials are another family of silica with large mesopores, synthesized using non-ionic block copolymer surfactants under highly acidic conditions.22 In addition to the co-operative pathways, nanocasting using already formed ordered mesoporous materials as hard templates has been developed to synthesize mesoporous materials.24 This method of nanocasting is highly effective for the synthesis of other non-siliceous porous oxide and carbon materials with ordered pore arrangements which are difficult to prepare directly by the surfactant-assisted route.25
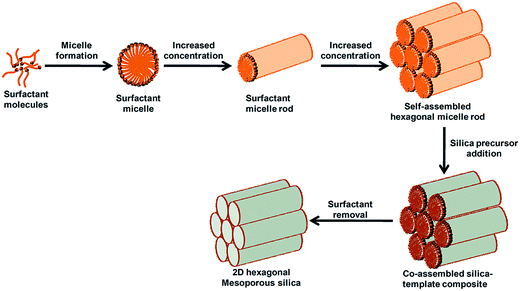 | ||
| Fig. 1 Surfactant assisted synthesis of mesoporous silica with a 2D ordered hexagonal pore arrangement. | ||
Non-siliceous mesoporous materials like oxides, mixed oxides,26 metal sulphides,27 metal phosphates,28 polymers,29 carbons30 and carbon nitrides31 have also been synthesized successfully using surfactant templating routes. The long range applications of these materials extend from gas adsorption, ion-exchange, magnetism, sensing, catalysis and electrochemistry to many versatile fields of research. Some of these mesoporous materials are purely inorganic; some are organic–inorganic hybrid materials while the others are completely organic based. Types of mesoporous solids commonly known in these three categories are shown in Table 1.
| Framework composition | Type of materials | Examples |
|---|---|---|
| Purely inorganic | Mesoporous silicas | MCM-41/MCM-48,8 SBA-15 (ref. 22) etc. |
| Metal containing mesoporous silicas | Ti-MCM-41, Zn–silica etc. | |
| Mesoporous metal oxides and mixed metal oxides | TiO2, AlO2, ZrO2, ZnTiO3 etc. | |
| Mesoporous metallophosphates | Silicotitanium phosphate, silicoalumino phosphate etc. | |
| Organic–inorganic hybrid | Periodic mesoporous organosilicas (PMOs) | Various metal containing PMOs etc. |
| Metal oxophenylphosphates | Iron phosphonate, chromium phosphonate etc. | |
| Purely organic | Mesoporous carbons | CMK-3 |
| Mesoporous polymers | Triazine based polymer, porphyrine based polymer |
3. Catalysis: homogeneous vs. heterogeneous
The phenomenon called ‘catalysis’ has been known since very ancient centuries, even though people knew nothing about the underlying mechanism and chemical process involved in it. The making of soap, the fermentation of wine to vinegar, and the leavening of bread are all processes involving catalysis. The name ‘catalysis’, first proposed in 1835 by Swedish chemist Jöns Jakob Berzelius (1779–1848), was taken from the Greek words kata, which means ‘down’ and lyein, meaning ‘loosen’. In a short paper summarising his concepts on ‘catalysis’ as a new force, Berzelius wrote: “It is, then, proved that several simple or compound bodies, soluble and insoluble, have the property of exercising on other bodies an action very different from chemical affinity. By means of this action they produce, in these bodies, decompositions of their elements and different recombination of these same elements to which they remain indifferent.” He called this new force a ‘catalytic force’.32 The actual basic role of a catalyst is to offer an alternative pathway of lower activation energy than the respective uncatalyzed reaction, resulting in a faster reaction rate without hampering the overall thermodynamics of the reaction. Enzymes are the most common and efficient catalysts found in nature. Not a single reaction in our body is possible without the aid of these biocatalysts. They have also been used as catalysts in food industries for many years. In the case of laboratory and industrial use, catalytic oxidation using oxygen, hydrogenation using metal catalysts, and many well known reactions are worthy of mention. Day by day the imperative role of catalysis in the manufacturing of the majority of chemicals used by our society is increasing rapidly. According to the survey report, in 2005, catalytic processes generated about $900 billion worth of products worldwide.Catalytic reactions are usually categorized as either homogeneous or heterogeneous. Biocatalysis (enzymatic reactions) is often considered as a separate group. A homogeneous catalysis reaction is one where both the catalyst, the reactants on which it works and the products, are all in the same phase (solid, liquid, or gas), generally in the liquid phase. The first formal example of this type is the reaction involving the decomposition of starch into glucose in boiling water in the presence of a sulphuric acid catalyst studied by the Russian chemist Gottlieb Sigismund Constantin Kirchhoff (1764–1833) in 1812. Both the sulfuric acid and the starch were in aqueous solution during the reaction.33 Organometallic catalysts are another example of homogeneous catalysts.34 On the other hand, heterogeneous catalysis is that where the catalyst is generally solid and remains as a distinct phase from the liquid or gaseous reaction media on which it acts. Most of the reactions that occur in industry are driven by heterogeneous catalysts. The role of finely divided iron in the Haber process for synthesis of ammonia or the role of vanadium oxide for the production of sulphuric acid in the contact process, etc. are well-known examples of this type. In fact, with the production of sulphuric acid in the “contact” process solved by Knietsch in the year 1898 in Europe, the idea of industrial catalysis was developed.35 Today porous materials are recognized as one of the most important materials in heterogeneous catalysis and they play the major role in industrial fine chemical synthesis.9
Homogeneous catalysis has some important properties like high selectivity of the products and good accessibility to catalytically active sites, etc. but with increasing public awareness regarding environmental issues, heterogeneous catalysis has become much more important over homogeneous catalysis.36 This is because some major problems like corrosion, toxicity, the difficulty of catalyst separation, recovery, regeneration and reuse, high cost and the creation of huge amounts of solid waste, etc. in the case of homogeneous catalysis, make it unsuitable for application in industrial purposes in this environmentally conscious and economically pressured world. Although homogeneous catalysts are used in some industries like the food, fine chemical, pharmaceutical and agrochemical industry etc., in this century heterogeneous catalysis has been proven to be the ultimate goal of industrial catalysis and in engineering fields. The industrial processes which are highly dependent on homogeneous catalysts are recommended to be used only by ‘heterogenization’, which means immobilization of the catalysts on a solid insoluble support which possesses all the properties of the homogeneous counterpart, and also the advantage of recyclability like in heterogeneous catalysis.37
Catalytic reaction on a heterogeneous support involves fundamental steps like diffusion and adsorption of the reactants onto a solid surface, successful reaction on it, then desorption of the products in bulk with regeneration of the catalyst for the next cycle. A schematic representation of a heterogeneous catalytic cycle taking place through different steps is shown in Fig. 2. Since the surface of catalyst plays a pivotal role in the related process, materials having high surface area are more desirable than nonporous materials to accomplish a significant part in industrial catalysis.1,9 Although most of the chemical industries generally use zeolites or activated carbon based microporous materials as a stable support for catalysis, the number of fascinating organic transformations successfully carried out over different mesoporous supports is growing very rapidly. In light of this a systematic brief discussion on various mesopore mediated important heterogeneous catalytic transformations under liquid phase reaction conditions has been made and illustrated in the following part of this review.
4. Heterogeneous catalysis over mesoporous materials
The most important feature of mesoporous materials for which they show great potential for catalytic applications, is the possibility of controlling the morphology and local environment at the catalytic site depending upon the requirements of a particular reaction. Basically the reasons for which mesoporous materials act as promising candidates for heterogeneous catalysis are, (i) they have a high surface area and narrow pore size distribution essential for acting as a catalyst bed, (ii) the dimension of the pores can be tuned by varying the functional groups and ligands to obtain good selectivity for the product of interest, (iii) they have good adsorption properties, which allow the facile diffusion of reactant and product molecules during reactions, (iv) the ability to vary the type and concentration of surface functionality to change the textural properties and (v) the ability to tailor the polarity/hydrophobicity/hydrophilicity of the surface in order to influence the yield of a catalytic reaction. Moreover, they are relatively non-toxic, non-corrosive, non-air sensitive, highly reusable, completely pollution-free, environmentally benign supports for catalytic transformation in the liquid phase.10 The role and function of mesoporous silica and other non-siliceous materials in mediating sustainable liquid phase reactions e.g. acid–base, redox, polymerization, condensation and other organic coupling reactions, etc. are discussed herein.4.1 Redox reactions over mesoporous materials
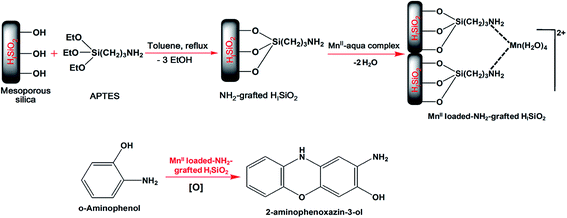
Although a pure mesoporous silica surface has high BET surface area (ca. 800–1400 m2 g−1) and a tunable pore diameter (2–50 nm), the material is not so effective for doing any catalytic reactions. By introducing one or more heteroatom and metal complex into the silica framework (metal doped silica) or often by suitable immobilization of organic functionalities (organosilica or organic–inorganic hybrid silicas) the desired catalytic activity of the material can be achieved. The advantage of choosing mesoporous silica as a support for incorporating other foreign atoms or functionalities is the inertness of the inorganic silica walls, where a tetravalent Si atom can be replaced by other reactive metal centers whilst retaining considerably good surface area and porosity.10 In spite of these benefits, silica structures like SBA-15, KIT-6, etc. have the limitation of losing porosity at elevated temperatures, which restricts their application in large scale heterogeneous catalysis. Metal incorporated mesoporous silica can be prepared using both a direct (or one step) or a post synthesis method.4 A number of related reports of mesoporous metal doped silica as heterogeneous catalysts can be mentioned in this respect, e.g. Zn–silica,20 Cr–MCM-41,37 CeO2–silica,38 Ti–MCM-41,39 Ce–Fe-SBA-15,40 Mn doped CeO2–silica,41 Pt and Rh nanoparticles supported silica,42 and so on. Periodic mesoporous silica (PMO), prepared by immobilizing organic moieties onto the inorganic silica surface by a direct synthesis method, can also act as a good redox catalyst when grafted with transition metals like V, Ti, Au, Mo, etc.43Further, additional advantages of mesoporous transition metal oxides and mixed oxide catalysts are their high crystallinity and also their capability of existing in various oxidation states due to having vacant d orbitals, which can take part in electron transfer from reactants during a given catalytic process.44 Thus, these metal oxides or metal doped silica based materials are attracting wide-spread attention in recent times as non-toxic and environmentally friendly mild catalysts replacing conventional homogeneous hazardous oxidants like chromate, permanganate or periodide. In the following section we summarize some redox reactions mediated by the mesoporous solids.
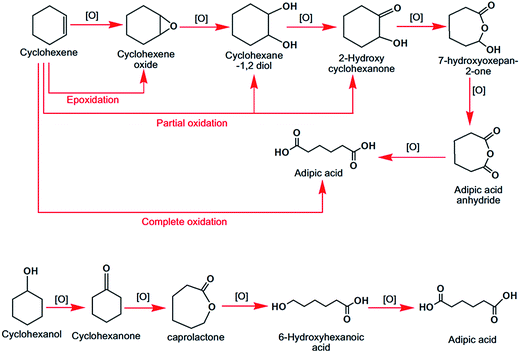 | ||
| Fig. 3 Different oxidation products of cyclohexene, cyclohexanol and cyclohexanone with adipic acid as the final product. | ||
Adipic acid is an industrially important dicarboxylic acid, which can be produced exclusively from the catalytic oxidation of cyclohexane, cyclohexene or cyclohexanone over mesoporous materials like WO3/SiO2,51 and the core–shell Fenton catalyst (Fe2O3/Al2O3)52 in the presence of H2O2 oxidant. In Fig. 3 the step by step formation of adipic acid from these hydrocarbons is shown. Over the core–shell Fenton catalyst, the process occurs through formation of a hydroxyl radical by the reaction of Fe3+ with H2O2, and that OH˙ helps to oxidize cyclohexanone to adipic acid through the intermediates caprolactone and 6-hydroxyhexanoic acid. Cr(VI) grafted mesoporous polyaniline, synthesized by radical polymerization of aniline with a Cr precursor, exhibits good conversion in the liquid phase oxidation of alkane and alkene in a non-toxic green solvent, water, with a reuse capability of more than five times.53 Highly selective oxidation of cyclohexane to produce cyclohexanone is a very challenging task since cyclohexanol is always obtained as a major side product. Very recently, another porous co-ordination polymer was reported by Zhang et al. who have employed atmospheric O2 as an oxidant.54 This catalytic pathway provides a useful route for the selective conversion of β-isophorone to ketoisophorone, a significant reaction for the synthesis of vitamin E.
Porous carbon based materials are also utilized as effective supports for the oxidation of hydrocarbons under liquid phase reaction conditions. N doped nanoporous carbon with supported Pd nanoparticles has shown high catalytic efficiency for the selective aerobic oxidation of indane, diphenyl methane and ethyl benzene etc. thus providing a potential route for large scale fine chemical synthesis.55 A new kind of boron- and fluorine-enriched polymeric mesoporous carbon nitride with high surface area has been prepared by Wang et al. who illustrate the potential of the material for selective cyclohexane to cyclohexanone conversion under mild conditions.56 This organic semiconductor with controlled mesopore structures and narrow pore size distribution greatly facilitates this selective oxidation reaction with reusability for several times without losing any activity. Heterogeneous organocatalysts are particularly high in demand as they are devoid of toxic metal and thus free from the possibility of metal-leaching during the catalytic reactions. In this context porous graphitic carbon nitride (g-C3N4) has shown high catalytic activity in the selective oxidation of many branched hydrocarbons under mild environmentally-friendly conditions.57,58
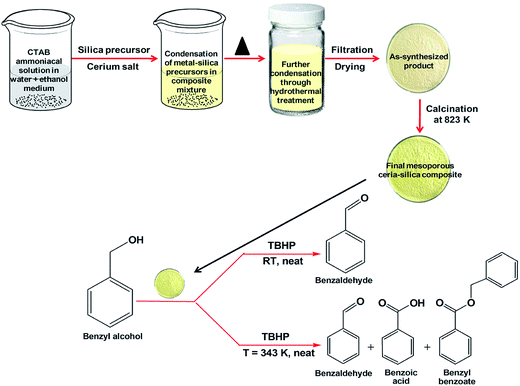 | ||
| Fig. 4 A schematic representation of the synthesis of a mesoporous ceria–silica composite and its catalytic application for oxidation of a primary alcohol (benzyl alcohol). | ||
The oxidation of sulfides to obtain sulfoxide and sulfones; products playing an imperative role in organic and biological reactions as well as in anti-hypertension and as cardiotonic agents etc., using industrially important heterogeneous and mesoporous catalysts replacing the homogeneous ones like halogen compounds, nitrates and transition metal oxides etc., has won great scientific interest.63 A Cr-grafted organic–inorganic hybrid mesoporous polymer, synthesized using an anionic surfactant, efficiently catalyzes the partial oxidation of different aromatic and aliphatic sulfides to their corresponding oxides with impressive yields, having excellent recyclability and no significant loss in activity.63 The almost selective oxidation of sulfide to sulfoxide using H2O2 oxidant in methanol solvent was reported over WO3 nanoparticles supported MCM-48 heterogeneous catalyst in the year 2005.64 The activity and stability of Mn and Cu oxo complexes have been increased to a great extent by ‘heterogenization’ of these homogeneous catalysts, i.e. by post-grafting onto mesoporous MCM-41. The resulting supported catalyst was able to convert methyl phenyl sulphide to sulfoxide with a high yield and exhibited excellent reusability up to five times in the presence of TBHP.65 Here, MnV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species present in the Mn–oxo salen complex stabilized in the mesoporous silica framework play the active role for this sulphide oxidation. Mesoporous graphitic carbon nitride (g-C3N4) polymer has been established as a green oxidation catalyst for the selective oxidation of various sulphides using visible light at room temperature.66
O species present in the Mn–oxo salen complex stabilized in the mesoporous silica framework play the active role for this sulphide oxidation. Mesoporous graphitic carbon nitride (g-C3N4) polymer has been established as a green oxidation catalyst for the selective oxidation of various sulphides using visible light at room temperature.66
Mesoporous oxides or mixed oxides can also act as a support similarly for thioether nanoparticles such as Au, Ni, Pt and Pd and these confined nanoparticles can catalyze the hydrogenation of aromatic nitro compounds. Mesoporous oxides like TiO2/Al2O3/SiO2/ZrO2 with confined PtPd/AuPd/AuPt thioether nanoparticles have been synthesized via a surfactant-assisted route and show highly selective synthesis of aniline from nitrobenzene under mild conditions using hydrazine hydrate as a hydrogenating agent.80 On the contrary, mesostructured nickel–aluminium mixed oxide hydrothermally synthesized using lauric acid as a structure directing agent, was itself efficient for the satisfactory reduction of nitrobenzene and its derivatives in 2-propanol solvent which acts as a hydride donor to generate substituted anilines.81 The synthetic pathway for the mesoporous self-assembled Ni–Al mixed oxide nanoparticles and their application for hydrogenation of nitrobenzene to aniline is shown in Fig. 7. Hydrogenation of D-glucose over Ru nanoparticles impregnated mesoporous hypercrosslinked polystyrene was reported by Sapunov et al.82 They also provided an elaborate mechanistic pathway and kinetics for the formation of D-sorbitol. In a non-toxic solvent like water, selective hydrogenation of phenol to cyclohexanone over ultra-small Pd nanoparticles grafted mesoporous carbon is a green synthesis in high demand. Pd supported N-functionalized ordered mesoporous carbon was prepared by nitration of a typical mesoporous carbon FDU-15 using NH3 followed by treatment with an aqueous solution of H2PdCl4.83 The catalyst was highly heterogeneous in nature and showed reusability up to 6 times with a good product yield each time.
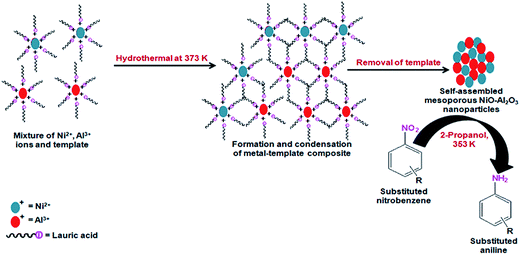 | ||
| Fig. 7 A schematic representation showing the synthesis of mesoporous Ni–Al mixed oxide and its application for hydrogenation of nitrobenzene to aniline. | ||
The above mentioned redox reactions are a few examples of a huge number of liquid phase catalytic reactions that have been carried out over the years using the mesoporous family of materials. In most of these cases the mechanisms of the reactions follow a free radical pathway mediated by one or two radicals generated in situ.53,74 In Table 2 we have summarized all the relevant reactions in tabular form for the convenience of the readers.
| Type of reactions | Catalysts | Reaction involved | Product yield (%) | Ref. |
|---|---|---|---|---|
| Oxidation of hydrocarbons | Ti doped PMO | Epoxidation of styrene, trans-stilbene norbonene, etc. | 65–80 | 45 |
| Cu-complex doped MCM-41 | Epoxidation of cyclohexene, cyclooctene, styrene, etc. | 92–99 | 46 | |
| Cu(II)-complex grafted SBA-16 | Partial oxidation of cyclohexene | 21–77 | 47 | |
| UO22+–MCM-41 | Allylic oxidation of α-pinene, β-pinene etc. | 80–88 | 48 | |
| Fe- & Ti-SBA-1 | Partial oxidation of styrene | 59–70 | 49 | |
| Au loaded mesoporous silica | Oxidation of cyclohexane | 10–34 | 50 | |
| Core–shell Fenton catalyst | Oxidation of cyclohexanone | 69 | 52 | |
| Cr–mesoporous polyaniline | Oxidation of alkane, alkene | 70–98 | 53 | |
| Porous co-ordination polymer | Conversion of β-isophorone to ketoisophorone | 85 | 54 | |
| Pd@N doped nanoporous carbon | Selective aerobic oxidation of indane, ethyl benzene | 14–30 | 55 | |
| Boron- and fluorine loaded mesoporous carbon nitride | Selective oxidation of cyclohexane to cyclohexanone | 2–8 | 56 | |
| g-C3N4 polymer | Oxidation of strained hydrocarbons | 26–100 | 57 and 58 | |
| Oxidation of alcohols | Au–ceria or iron oxide–HMS | Oxidation of 1-octanol | 10–79 | 59 |
| Mesoporous CeO2–silica | Oxidation of benzyl alcohol | 51 | 37 | |
| Mesoporous Cr2O3–PMA composite | Oxidation of 1-phenylethanol | 85–87 | 60 | |
| Iron oxide on mesoporous carbon (FeOx/H-CMK-3) | Oxidation of benzyl alcohol to benzaldehyde | 72 | 61 | |
| Pd@silica core–shell nanosphere | Solvent-free aerobic oxidation of long chain primary alcohols | 10–61 | 62 | |
| Oxidation of sulfides | Cr–mesoporous polymer | Partial oxidation of different aromatic and aliphatic sulfides | 7–97 | 63 |
| WO3 NP–MCM-48 | Oxidation of different sulfides | 100 | 64 | |
| Mn and Cu oxo complex–MCM-41 | Oxidation of methyl phenyl sulphide | 65–100 | 65 | |
| Mesoporous g-C3N4 polymer | Selective oxidation of sulphides | 39–98 | 66 | |
| Ammoximation | Ti-grafted ethane bridged porous inorganic–organic hybrid silica | Oxidation of cyclohexanone, cyclodecanone | 42–90 | 71 |
| Oxidation of amines | Mesoporous cobalt oxide–silica composite | Oxidation of aniline and its derivatives | 26–100 | 67 |
| Ti, Zr-doped mesoporous silica | Oxidation of aniline | 100 | 68 | |
| Mn, Cu, Co & Zn-NH2-grafted organic–inorganic hybrid silica | Oxidation of amine | 90–100 | 69 | |
| Hydroxylation of aromatics | Fe incorporated mesoporous silica | Hydroxylation of phenol | 20–50 | 72 |
| Ferrocene loaded nanocomposite of mesoporous polymer and silica | Conversion of phenol to form dihydroxybenzene | 25–30 | 73 | |
| VO(acac)2 incorporated PMO | Selective conversion of benzene to phenol | 12–38 | 74 | |
| FeCl3/g-C3N4 | Conversion of benzene to phenol | 38 | 75 | |
| Mesoporous Zn doped aluminophosphate | Selective hydroxylation of benzene to phenol | 99 | 76 | |
| Hydrogenation & other reductions | Chiral Ir and Ru complexes-loaded mesoporous silica | Asymmetric hydrogenation of aryl substituted ketones and quinolones | 80–99 | 78 and 79 |
| PtPd/AuPd/AuPt-embedded TiO2/Al2O3/SiO2/ZrO2 | Selective conversion of nitrobenzene to aniline | 65–87 | 80 | |
| Mesostructure NiO–Al2O3 | Reduction of nitrobenzene and its derivatives | 36–57 | 81 | |
| Ru–mesoporous polystyrene | Hydrogenation of D-glucose | 40–43 | 82 | |
| Pd nanoparticles-loaded mesoporous carbon | Selective hydrogenation of phenol to cyclohexanone | 23–80 | 83 |
4.2 Acid–base reactions mediated by mesoporous materials
Lewis and Brønsted acid or basic sites present in a solid catalyst are the main reason for the generation of the acid–base properties in a given solid. The Lewis acidity originates due to the presence of metal ions coordinated to the mesoporous support whereas –COOH, –SO3H, –NR3+ groups etc. are generally responsible for the Brønsted acidity. In the same way, –NH2, –NMe2 etc. and –COO− groups account for Lewis and Brønsted basicity, respectively. The number and strength of these sites determine the catalytic activity of the concerned materials and also their selectivity for the organic transformations. Moreover, surface properties i.e. huge surface area and pore width of the mesoporous material give an additional advantage compared to the other nonporous materials. Though inherent weak acid sites restrict the application of mesoporous materials in many petrochemical reactions, they still demonstrate good potential for the reactions which require a lower level of acidity, e.g. Friedel–Crafts alkylation of organics.10 A lot of scientific reports such as those on mesoporous perovskite ZnTiO3,19 Zn–silica,20 –COOH grafted functionalized mesoporous silica,84 Mn-doped ceria–silica,42 sulphonated zinc phosphonate,85 zirconium oxophosphate,86 and so on have been published in the literature regarding the acid–base catalytic reactions over mesoporous supports. In this section we shall illustrate some of those heterogeneous reactions carried out in the liquid phase. | ||
| Fig. 8 Friedel–Crafts products obtained from various substrates during reactions over different mesoporous catalysts. | ||
FC reactions are also very common over mesoporous silica based materials as revealed by many recent reports. A 3D network of mesoporous gallosilicate (GaSBA-1) was prepared in an acidic medium using the cationic surfactant CTAB and an appropriate amount of Ga salt. This material has been tested for the benzylation of various aromatics using benzyl chloride. The catalytic role of the Ga species along with a schematic representation of a suitable mechanism has been mentioned and explained by the authors.92 A Cu(II) complex containing a chiral bis(oxazoline) ligand has been immobilized on mesoporous silica and the catalyst was employed for asymmetric benzoylation of 1,2-diphenyl-1,2-ethanediol which produces a highly enantioselective product using benzoyl chloride under mild conditions.93 There are many other reports on the alkylation of aromatics using alcohols over metal doped mesoporous silica. Sulphated Zr–MCM–41 and Zr–MCM–48 solid acid catalysts have successfully catalyzed attached substituent of phenol aromatic ring in presence of tert-butyl alcohol to produce 4-tert-butyl phenol as major product with above 90% yield at 413 K temperature.94
 | ||
| Fig. 9 (A) Esterification and (B) transesterification reactions for biodiesel production by acid or base catalysts. | ||
Both Lewis and Brønsted acids are used as catalysts for the esterification of long chain fatty acids and the equilibrium of the reversible reaction can be right shifted towards the ester conversion by removing water from the reaction medium through azeotropic distillation or using molecular sieves or using an excess of alcohol.19 Replacing previously used strong Brønsted acids like H2SO4, p-TsOH (p-toluenesulphonic acid) various environmentally non-hazardous, recyclable functionalized mesoporous materials have been designed, which are employed successfully as heterogeneous catalyst for the biodiesel production. The mechanism of an esterification reaction over solid acid catalysts can proceed via activation of the carboxylic acid by the catalyst, then nucleophilic attack by the alcohol on the activated carbonyl carbon to form a tetrahedral intermediate and finally formation of an ester with elimination of one molecule of water.19 Esterification of a long chain fatty acid with alcohol and vice versa is observed over mesoporous zirconium oxophosphate material which not only produces the corresponding ester in high yield but also shows high reusability up to the 5th cycle, the catalytic activity being attributed to the large surface area and large number of acidic sites located at the surface of the phosphate material.86 In Fig. 10, a simple scheme for the synthesis of this Zr-oxophosphate material is shown along with its application in the proposed mechanistic pathway for an acid catalyzed reaction. The presence of the acidic sites necessary for catalytic activity in the Zr-catalyst has been investigated and quantitatively estimated by using the temperature-programmed desorption of ammonia (NH3-TPD) measurement.86 The TPD result suggested that with the increase in surface Brønsted acidity a higher yield of the biodiesel products was obtained. Esterification over periodic mesoporous silica materials has been described in the review published by Yang et al. in 2009.96 MCM-48 supported tungstophosphoric acid acts as a heterogeneous catalyst for esterification of a number of fatty acids like palmitic, lauric, stearic, isostearic, myristic etc. with alcohols, e.g. cetyl, butanol, hexanol, octanol etc. in 11 MPa super critical CO2 medium at a temperature of 373 K to produce esters in an appreciable amount which increases with the increasing chain length of the fatty acids.97
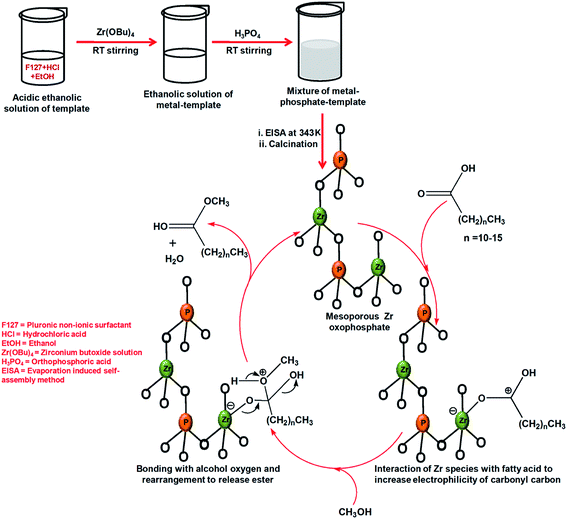 | ||
| Fig. 10 A simple schematic pathway of the synthesis of mesoporous zirconium oxophosphate and a proposed catalytic cycle for the esterification reaction over this catalyst. | ||
Transesterification reactions involve an acid or base mediated reaction where increasing the electrophilicity of a carbonyl carbon of fatty acids or the nucleophilicity of an attacking alcohol can improve the reaction rate considerably.95 Sulphonated porous zinc phosphonate is a example of a green catalyst, which provides an eco-friendly route for biodiesel production via transesterification of long chain fatty acids in the presence of methanol at room temperature.85 Thus, ordered Zn-doped mesoporous silica20 or amine-functionalized mesoporous organosilica98 or mesoporous polyoxometalate–tantalum pentoxide composite, H3PW12O40/Ta2O5 (ref. 99), are very active acid catalysts to give a satisfactory yield of the respective esters under suitable conditions. In the mesoporous H3PW12O40/Ta2O5 composite, (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TaOH2)n+[H3−nPW12O40]n− species formed at the catalyst surface of the composite via a Ta–O–W bond facilitates the electron transfer from the terminal oxygen atoms of W
TaOH2)n+[H3−nPW12O40]n− species formed at the catalyst surface of the composite via a Ta–O–W bond facilitates the electron transfer from the terminal oxygen atoms of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups to Ta2O5; encouraging a good amount of proton release to ensure high Brønsted acidity of the catalyst responsible for the biofuel conversion.99 On the contrary, transesterification using base catalysts has been efficiently carried out over Mn-doped ceria–silica and MgO-functionalized mesoporous silica. etc. Mn doped ceria–silica catalyzes the transesterification of methyl benzoate, ethyl cyanoacetate and ethyl chloroacetate with methanol, butanol and octanol under solvent-less mild reaction conditions. The presence of basic sites in the MgO–silica was determined by CO2 sorption measurements and the sites help to produce biofuel from vegetable oil in the presence of ethanol, achieving a conversion level of above 90% within 5 h reaction time.100
O groups to Ta2O5; encouraging a good amount of proton release to ensure high Brønsted acidity of the catalyst responsible for the biofuel conversion.99 On the contrary, transesterification using base catalysts has been efficiently carried out over Mn-doped ceria–silica and MgO-functionalized mesoporous silica. etc. Mn doped ceria–silica catalyzes the transesterification of methyl benzoate, ethyl cyanoacetate and ethyl chloroacetate with methanol, butanol and octanol under solvent-less mild reaction conditions. The presence of basic sites in the MgO–silica was determined by CO2 sorption measurements and the sites help to produce biofuel from vegetable oil in the presence of ethanol, achieving a conversion level of above 90% within 5 h reaction time.100
Mesoporous carbon can act as a good support for metal nanoparticles or metal oxides to improve catalytic performances, e.g. Mg–Zr mixed oxide supported on mesoporous carbon catalyzes the cross-aldol reaction of furfural and acetone followed by hydrogenation–dehydration to form C8 and C13 alkanes at a temperature of 323 K, the conversion and selectivity being higher than when bulk Mg–Zr mixed oxide is used.107 The reaction mechanism and the kinetic model of aldol condensation over the distributed basic sites of this supported catalyst are also explained by the authors. Bai et al. have synthesized mesoporous γ-Al2O3 based on a cation–anion double hydrolysis method and the material was proven to be truly efficient for the aldol reaction of cyclohexanone carried out in a water-separating device in the form of water–cyclohexanone azeotrope at a temperature of 438 K for 2 h when the conversion was as high as ∼70%. The remarkably high catalytic activity of crystalline γ-Al2O3 could be attributed to the huge surface area, precise mesopore diameter, absence of anionic species as well as the surface basicity generated due to aluminium and oxygen vacancies.108 Based on the experimental results they also proposed a suitable mechanism for the formation of α,β-unsaturated ketone involving the absorption of α-H of cyclohexanone by the aluminium vacancies. Aldol condensation of benzaldehyde and heptanal to form jasminaldehyde under solvent free conditions is reported over novel Mg–Al mixed oxide supported on hexagonal mesoporous silica (Fig. 12).109 The bi-functional behaviour of this catalyst, where the weak acid sites activate the aldehyde by protonation and the basic sites favour the formation of the enolate heptanal intermediate, helps in carrying out the reaction successfully.109 In this context a completely metal-free organocatalyst has been developed via heterogenizing (S)-proline on MCM-41, which catalyzes the asymmetric aldol condensation of p-nitrobenzaldehyde and 2,2-dimethyl-1,3-dioxan-5-one under the influence of different solvents ranging from hydrophobic to hydrophilic, proving hydrophilic polar media as more suitable for catalysis.110
Mesoporous CexZr1−xO2 solid consisting of nanometer sized particles was synthesized via a sol–gel method and it showed excellent chemoselectivity for the classical Knoevenagel condensation of benzaldehyde and malononitrile owing to the presence of pores of a large size (∼10 nm) and both surface acidic and basic sites.111 The Knoevenagel reaction under mild conditions is also reported over ordered mesoporous Ni–Al mixed oxide obtained from Ni–Al-layered double hydroxides (LDHs) by ultrasonic irradiation using Pluronic-F127 surfactant. The catalyst exhibits high selectivity for the product with good reusability without significant loss of activity.112 Mesoporous oxides without basic sites can also be used for Knoevenagel reaction after proper functionalization of the oxide surface with amine groups. By co-condensation of 3-aminopropyltrimethoxy silane and zirconium precursors in CTAB surfactant solution, mesoporous amine grafted zirconia can be prepared and used for condensation of benzaldehyde and a malonic ester, diethyl malonate, in methanol solvent at room temperature to produce cinammic acid exclusively.113 Besides this, as metal-free organocatalysts, guanidine base immobilized on mesoporous silica SBA-15 and urea embedded mesoporous polymer are highly recommended for Knoevenagel reaction.114,115 Two types of Knoevenagel condensation, one between benzaldehyde and ethyl cyanoacetate, and another between cyclohexanone and benzylcyanide have been reported over basic guanidine functionalized silica at RT as well as at an elevated temperature.114 On the contrary, mesoporous urea-functionalized polymers synthesized through the non-ionic F127 surfactant-directed urea–phenol–formaldehyde oligomer self-assembly approach have been utilized for Knoevenagel reaction of aromatic aldehyde and ethyl cyanoacetate at mild temperatures in a non-hazardous aqueous medium. In Fig. 13, a simple pictorial representation for the synthesis of this mesoporous urea-polymer along with a proposed mechanism for the catalytic reaction over this polymer is shown. The synergic effect between the urea active sites embedded on the mesopore wall and their neighbouring surface phenolic –OH groups in the mesoporous support accounts for the success of this reaction along with the good stability of the catalyst inhibiting the leaching of active species as documented through successful reuse up to six reaction cycles.115
| Type of reactions | Catalysts | Reaction involved | Product yield (%) | Ref. |
|---|---|---|---|---|
| Friedel–Crafts reaction | Mesoporous perovskite ZnTiO3 | Reaction of p-xylene, toluene, benzene with benzyl chloride | 90–93 | 19 |
| Mesoporous iron phosphate | Benzylation of benzene, toluene, p-xylene | 100 | 88 | |
| Silicotungstic acid-loaded mesoporous alumina | Benzoylation of anisole with p-toluoyl chloride | 80–100 | 89 | |
| Mesoporous sulphated Nb oxide | Reaction of toluene and anisole with benzyl alcohol | 100 | 90 | |
| Mesoporous Mo-doped carbon sphere | Alkylation of toluene using benzyl alcohol | 100 | 91 | |
| Mesoporous GaSBA-1 | Benzylation of various aromatics using benzyl chloride | 95–100 | 92 | |
| Cu(II) complex–bis(oxazoline) loaded mesoporous silica | Asymmetric benzoylation of 1,2-diphenyl-1,2-ethanediol | 29–40 | 93 | |
| Sulphated Zr–MCM-41 and Zr–MCM-48 | Reaction of phenol with tert-butyl alcohol | 18–91 | 94 | |
| Esterification | Mesoporous ZnTiO3 | Reaction of palmitic, lauric, oleic acid with methanol | 73–92 | 19 |
| Mesoporous zirconium oxophosphate | Esterification of long chain fatty acid with alcohol and vice versa | 46–91 | 85 | |
| Tungstophosphoric acid-loaded MCM-48 | Esterification of palmitic, lauric, stearic, isostearic, myristic acids with different alcohols | 48–97 | 97 | |
| Trans-Esterification | Sulphonated porous zinc phosphonate | Transesterification of long chain fatty acids with methanol | 35–81 | 84 |
| Zn-doped mesoporous silica | Transesterification of ECA, EClA, EAA, EA etc. | 25–94 | 20 | |
| Amine-grafted OMS | Transesterification of glyceryl tributyrate with methanol | 75–95 | 98 | |
| H3PW12O40/Ta2O5 porous composite | Reaction of palmitic, oleic, linoleic acid with methanol | 20–68 | 99 | |
| MgO-grafted mesoporous silica | Vegetable oil with ethanol | 72–96 | 100 | |
| Acetalization reactions | Mesoporous silica | Acetalization of cyclohexanone with methanol | 87–89 | 103 |
| Mesoporous sulphated zirconia | Reaction of cyclohexanone with methanol, ethylene glycol | 72–98 | 101 | |
| Ni–Zr supported mesoporous carbon | Reaction of acetone and glycerol | 68–100 | 104 | |
| Mesoporous SO3H-loaded organosilica | Reaction of heptanal with 1-butanol | 39–97 | 105 | |
| Aldol condensation | Mg–Zr supported mesoporous carbon | Cross-aldol reaction of furfural and acetone | 98 | 107 |
| Mesoporous γ-Al2O3 | Homo aldol reaction of cyclohexanone | 60–80 | 108 | |
| Mg–Al mixed oxide loaded HMS | Condensation of benzaldehyde and heptanal | 100 | 109 | |
| (S)-Proline loaded MCM-41 | Reaction of p-nitrobenzaldehyde & 2,2-dimethyl-1,3-dioxan-5-one | 20–96 | 110 | |
| Knoevenagel condensation | Mesoporous CexZr1−xO2 | Condensation of benzaldehyde and malononitrile | 72–90 | 111 |
| Mesoporous Ni–Al mixed oxide | Reaction of benzaldehyde and malononitrile | 99 | 112 | |
| Mesoporous amine grafted zirconia | Condensation of benzaldehyde with diethyl malonate | 80–98 | 113 | |
| Guanidine base loaded SBA-15 | Condensation of cyclohexanone with benzyl cyanide | 30–65 | 114 | |
| Urea embedded porous polymer | Condensation of benzaldehyde with ECA, EAA, etc. | 21–99 | 115 | |
| Baeyer–Villiger oxidation | Mesoporous Mg–Al mixed oxide | BV oxidation of cyclohexanone, cyclooctanone, methyl isopropyl ketone, ethyl methyl ketone | 62–89 | 117 |
| Metalloporphyrin-bridged PMO | Selective conversion of cyclohexanone to ε-caprolactone | 10–100 | 118 | |
| Mesoporous Zr-phosphate | Conversion of cyclic ketones like adamantanone to lactones | 96 | 119 | |
| Beckmann rearrangement | Al doped MCM-41 | Cyclohexanone oxime | 46–50 | 121 |
| SO3H-grafted SBA-15 | Cyclohexanone oxime to ε-caprolactam | 14–52 | 122 |
4.3 Different coupling reactions over mesoporous catalysts
The involvement of mesoporous solids for hydrosilylation, cyanosilylation, Diels–Alder, Suzuki reactions etc. in mediating C–C, C–N, C–H, C–S and C–O coupling and cross coupling reactions in heterogeneous media to synthesize various value-added organic fine chemicals is a widely recognized area of research nowadays. Transition metals like Cu, Ni, Fe etc. or noble metals like Au, Pt, Pd etc. supported mesoporous siliceous and non-siliceous materials, polymers or carbons, play an imperative role in these types of organic transformations.123 A concise report on all these reactions is mentioned in the following sections and these are also summarized in Table 4.| Type of reactions | Reaction name | Reaction involved | Catalysts used | Product yield (%) | Ref. |
|---|---|---|---|---|---|
| C–C coupling reaction | Sonogashira coupling | Reaction of iodobenzene & phenylacetylene | PdNP loaded mesoporous starch | 90–99 | 126 |
| Substituted aryl halides with phenylacetylene | Pd-grafted triazine functionalized mesoporous polymer | 60–90 | 125 | ||
| Reaction of substituted aryl halides & phenylacetylene without Cu co-catalyst | Pd-loaded periodic mesoporous silica | 72–90 | 124 | ||
| Reaction of substituted benzoyl chloride and phenyl acetylene | Mesoporous tin silicates | 76–97 | 127 | ||
| Suzuki or coupling | Reaction of substituted aryl halide with sodium salt of phenyltrihydroxyborate | Pd-incorporated triazine functionalized mesoporous polymer | 60–95 | 125 | |
| Reaction of substituted aryl halides with substituted phenylboronic acids | Pd(II) complex anchored 2D-HMS | 63–99 | 128 | ||
| Reaction of different aryl halides with phenyl boronic acids | Pd@imidazolium-functionalized SBA-15 | 87–99 | 129 | ||
| Substituted aryl halides with substituted phenyl boronic acids | PdNP@mesoporous carbon | >99 | 130 | ||
| Coupling of substituted aryl halides with different substituted phenyl boronic acids | Pd grafted mesoporous ionic-liquid framework organosilica | 55–99 | 131 | ||
| Coupling of substituted aryl halides with different substituted phenyl boronic acids | Au Schiff-base complex anchored mesoporous support | 84–95 | 132 | ||
| Heck reaction | Coupling of substituted aryl halides with acrylic acid or styrene | Pd loaded mesoporous polymer | 50–95 | 125 | |
| Coupling of substituted aryl iodides with styrene | Pd@mesoporous triallylamine polymer | 88–95 | 133 | ||
| Reaction of substituted aryl iodides with substituted olefins | Pd supported TMG ionic liquid modified SBA-15 | 60–94 | 134 | ||
| Reaction of substituted aryl halides (Cl, Br, I) with Styrene | Pd supported NiFe2O4 | 72–97 | 135 | ||
| Hiyama coupling | Cross couplings of various substituted aryl halides with alkyl and aryl trimethoxysilane | Pd loaded phloroglucinoldiimine modified PMO | 65–95 | 124 | |
| Substituted aryl halides with different trimethoxysilane | Pd NP loaded SBA-15 | 70–95 | 136 | ||
| Ullmann reaction | Reaction of iodobenzene to form biaryl | Pd doped Ph-functionalized MCM-41 | 70–88 | 137 | |
| Homo coupling of chlorobenzene in water | Pd@mesoporous silica–carbon nanocomposites | 100 | 138 | ||
| Reaction of various aryl iodides | AuNP@phenylene containing PMO | 80–95 | 139 | ||
| Stille reaction | Organotin compound with a sp2-hybridized organic halide | Pd loaded triazole & benzene grafted PMO | 60–80 | 140 | |
| Negishi coupling | Cross coupling of an organozinc compound with an organic halide | — | — | 141 | |
| C–S coupling reaction | — | Reaction of 4-chlorothiophenol & substituted aryl iodides | Mesoporous NiO–ZrO2 nanocrystals | 39–89 | 145 |
| Reaction of thiols with different aryl halides | Mesoporous Cu–Fe-hydrotalcite | 50–90 | 143 | ||
| S-arylation of aryl iodides with thiols | CuO well-dispersed on mesoporous silica | 66–97 | 147 | ||
| Reaction of substituted aryl iodide with thiophenol | Cu(OAc)2 loaded mesoporous furfural functionalized silica | 75–88 | 144 | ||
| Coupling of aryl halides, benzyl bromides with thiourea | Cu-grafted imine functionalized SBA-15 | 80–88 | 146 | ||
| Three component coupling | Biginelli condensation | One pot reaction of aryl aldehyde, EAA and urea | Al–MCM-41 | 68–89 | 152 |
| Reaction of substituted aryl aldehyde, EAA and urea | Fe3O4 nanoparticles@cysteine loaded SBA-15 | 78–85 | 153 | ||
| One pot reaction of aryl aldehyde, EAA and urea | Phosphonic acid functionalized 2D HMS | 83–92 | 154 | ||
| A3 coupling | Coupling of aldehyde, alkyne and amine in glycol | AgNP doped mesoporous SBA-15 | 40–95 | 150 | |
| Three component reaction of aldehyde, alkyne and amine | Oxidized Cu NP supported on titania | 50–88 | 149 | ||
| Coupling of substituted aryl aldehyde, substituted phenylacetylene with different amine bases | Au@ionic liquid framework PMO heterogeneous support | 75–88 | 151 |
The Sonogashira coupling reaction is a highly demanding reaction in organic synthesis, which is usually catalyzed by Pd metal to form a C–C bond between a terminal alkyne and an aryl or vinyl halide in the presence of an amine base and with or without a Cu co-catalyst.124 Instead of using Pd metal directly, Pd-grafted mesoporous silica, polymer or carbons are highly appreciated as non-corrosive, stable, environmentally-friendly, highly reusable catalysts for the production of aryl or vinyl substituted alkynes using the Sonogashira reaction. Using Cu catalyst the mechanism is initiated with the simultaneous formation of copper acetylide complex when the copper ion catalyst attacks on the terminal alkyne in presence of base and oxidative addition of organic halides to Pd(0) occurs to form a Pd(II) intermediate complex. The Pd(II) intermediate on reaction with copper acetylide followed by reductive elimination gives the final C–C coupled product with regeneration of the Pd(0) catalyst.125 The reaction is reported to be highly successful over a Pd-grafted triazine functionalized ordered mesoporous polymer material125 and a Pd nanoparticles loaded mesoporous polysaccharide-derived material,126 etc. The catalysis in the former case (Pd–polysaccharide) was carried out without a Cu co-catalyst under microwave conditions at a temperature of 403 K, while the latter (Pd–triazine polymer) employs a much lower temperature (363 K) and a green solvent, water, for the coupling of substituted aryl halides with phenylacetylene in the presence of triethanol amine and a Cu catalyst. A similar experiment was carried out very efficiently by Modak et al. with a Pd-loaded periodic mesoporous silica catalyst in aqueous media at a temperature of 393 K using hexamine base and without any Cu catalyst.124 The yield was very satisfactory in this Cu-free Sonogashira reaction and a suitable mechanism has also been proposed showing the coupling between a Pd(II) intermediate and an alkyne to form an alkyne co-ordinated Pd complex which was followed by the attack of a base producing the final product with regeneration of the catalyst.124 A comparative mechanism of Sonogashira coupling without and with a Cu co-catalyst over a Pd@PMO material and a mesoporous Pd@triazine polymer, respectively, has been proposed in Fig. 15. An extraordinary approach was made by Reddy et al. who tested an acyl Sonogashira coupling reaction using acyl halide (instead of aryl halide) over a mesoporous tin silicate material (an alternative to a Pd catalyst) synthesized by a simple surfactant assisted soft-templating route.127 The reaction was performed at room temperature under solvent-free conditions using triethyl amine as a base as well as a solvent and the catalyst was highly stable for reuse for several cycles to produce a high yield of ynones.
 | ||
| Fig. 15 A comparative mechanism proposed for Sonogashira coupling without and with a Cu co-catalyst over Pd@PMO and mesoporous Pd@triazine polymer, respectively. | ||
The Suzuki or Suzuki–Miyuara cross coupling reaction first published by Akira Suzuki in 1979 is the organic reaction of an organoboronic acid with an aryl or alkyl halide in the presence of Pd(0) to form a C–C coupled product with or without using a base.124 Compared to the homogeneous counterparts, Pd-grafted mesoporous catalysts provide ease of product separation, catalyst recovery by simple filtration and excellent recycling efficiency. Recently, a number of reports have been revealed regarding this Suzuki coupling reaction studied in the presence of Pd immobilized heterogeneous mesoporous silica or polymer using mild reaction conditions. The reaction proceeds through oxidative addition of a halide to Pd(0) to form an organopalladium(II) halide. This species on reaction with an organic base followed by a nucleophilic attack by a boronate species via transmetalation forms an organopalladium(II) species. Finally, following reductive elimination of the desired biaryl or divinyl the original palladium(0) catalyst is restored.128,129 Ordered mesoporous carbon with loaded Pd nanoparticles has also been utilized for a Suzuki coupling reaction in a DMF–water solvent at a temperature of 423 K (Fig. 16).130 A base free route is suggested using a sodium salt of a boronic species instead of an organoboron compound (Fig. 16). Karimi et al. have carried out the reaction with Pd-grafted mesoporous ionic-liquid framework organosilica in aqueous media in the presence of K2CO3 under very mild conditions.131 The reaction is very successful at room temperature giving a satisfactory yield. In addition to Pd, Au metal anchored mesoporous support also has high efficiency and true heterogeneity for Suzuki homo coupling reactions under mild conditions, reports of which are not quite rare in the literature.132
 | ||
| Fig. 16 Suzuki–Miyaura cross coupling reactions (A) with K2CO3 base over Pd doped ordered mesoporous carbon and (B) without base over Pd grafted mesoporous triazine polymer. | ||
Another example of C–C cross coupling is the Heck or Mizoroki–Heck reaction involving an interaction between an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst or a palladium thioetherific-tethered catalyst to form a substituted alkene via C–C bond formation. Allowing substitution on a planar system, the reaction is very much important in organic synthesis with applications ranging from natural product and bioactive compound synthesis to the development of organic electronics.133 Bases used in this reaction are generally triethylamine, potassium carbonate and sodium acetate, etc. The reaction occurs through the oxidative addition of a halide with a Pd(0) species to form a Pd(II) halide intermediate, then formation of a π-complex with an alkene, next some rearrangement and β-elimination to form a new palladium-alkene π-complex and finally reductive elimination to give the final product with regeneration of the catalyst.133 The reaction in a non-hazardous water–ethanol mixture using a Pd loaded mesoporous polymer shows a high percentage of conversion in a very short time,125 however there is always a positive effort to use more green solvents like water for such a reaction and a bit lower reaction temperature. Mondal et al. have successfully employed a Pd-loaded triallylamine based mesoporous polymer catalyst for the Heck coupling reaction.133 The scheme in Fig. 17 illustrates a simple route for the synthesis of this Pd-based mesoporous polymer and a Heck coupling reaction over this catalyst. Another convenient and environmentally friendly method is the reaction in solvent-free conditions as reported by Ma et al. They employed a highly reusable catalyst, which consisted of Pd supported on 1,1,3,3-tetramethylguanidinium (TMG) ionic liquid modified mesoporous SBA-15, for Heck coupling of different aryl halides with substituted olefins at a temperature of 413 K to produce C–C coupled arylated olefins. The yield was high enough with respect to the reaction time and the amount of catalyst showing excellent activity for several reaction cycles without significant metal leaching.134 On the contrary, Pd supported on NiFe2O4 catalyst, highly efficient for Mizoroki–Heck reactions of chloro, bromo and iodo substituted aryl halides with styrene in DMF solvent, has the advantage of magnetic separation from the reaction mixture.135
Hiyama coupling, another palladium-catalyzed cross-coupling reaction used in organic natural product synthesis involves reaction of organosilanes with organic halides to form a carbon–carbon bond (C–C bond) in the presence of an activating agent such as a fluoride ion or a base. The use of an expensive fluoride source and hazardous organic solvents like DMF or DMSO etc. has recently been modified by Modak et al. who suggested a suitable mechanistic pathway for the Hiyama coupling reaction without the need for any fluoride source at a moderate temperature over a newly developed Pd-grafted PMO.124 They used NaOH to maintain the alkaline conditions and water as a solvent to make the process more environmentally-friendly. The heterogeneity of the Pd-catalyst was proven by different tests like a hot filtration test, three phase test and a solid phase poisoning test which showed minimum metal leaching with retention of catalytic activity. Recently, another interesting catalyst is a Pd nanoparticles loaded SBA-15 material fabricated through deposition of Pd nanoparticles in the micropores of SBA-15, consisting of hydrophobic trimethylsilyl (TMS) or triphenylsilyl (TPS) group functionalized mesopores.136 In Fig. 18, we have shown a scheme for the synthesis of this novel catalyst. This nanocatalyst shows excellent activity for the Hiyama cross-coupling between various aryl triethoxysilanes and aryl halides under relatively mild reaction conditions (373 K) to form different biphenyl derivatives.
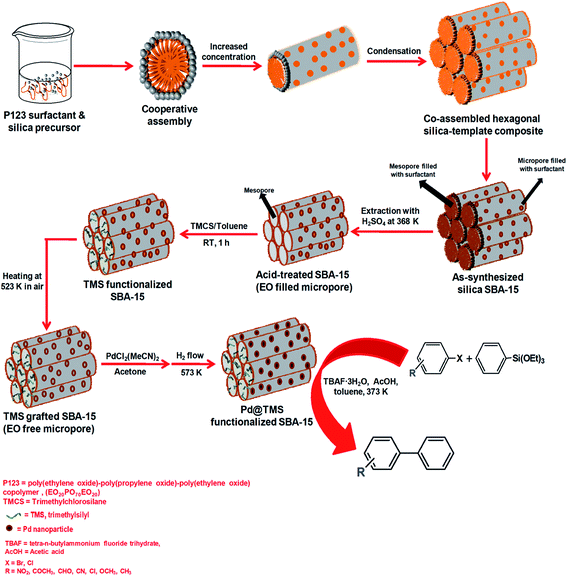 | ||
| Fig. 18 A simple synthesis scheme for Pd nanoparticles doped trimethylsilyl group functionalized SBA-15 and the Hiyama coupling reaction over this material. | ||
The Ullmann reaction, named after scientist Frietz Ullmann, is a homo coupling reaction of aryl halides to form a symmetric biaryl derivative in the presence of Cu, Pd or Au catalysts (Fig. 19). A number of reports have been published about the Ullmann reaction in aqueous media to provide a green route for the synthesis of biphenyls over heterogeneous catalysts. Ullmann reaction of iodobenzene to form a biaryl with a maximum ∼80% yield and high selectivity in water as a solvent was carried out over a novel Pd doped Ph and Al-functionalized MCM-41 catalyst.137 Coupling of substituted chlorobenzene over heterogeneous palladium catalysts supported on ordered mesoporous silica–carbon nanocomposites was carried out both at normal and elevated temperatures showing product formation increasing with temperature.138 The reaction is also catalyzed by a novel recyclable catalyst, comprising Au nanoparticles embedded bifunctional phenyl containing periodic mesoporous organosilica (Au@PMO),139 with an unexpected high yield (above 90%) and negligible leaching of metal (Fig. 19).
The Stille reaction, or Migita–Kosugi–Stille coupling, used extensively in organic synthesis is the reaction of an organotin or organo stannanes compound with an sp2-hybridized organic halide catalyzed by palladium usually performed under an inert atmosphere (Fig. 19). The mechanism involves three basic steps like in the coupling reactions: oxidative addition, transmetalation and reductive elimination. A highly efficient, reusable Pd mesoporous catalyst reported by Singh et al. was synthesized by grafting a Pd anchored triazole ligand onto the surface of a functionalized benzene containing periodic mesoporous organosilica with a uniform hexagonal arrangement prepared under basic conditions using CTAB surfactant.140
Beside these reactions, there are also Negishi coupling involving C–C cross coupling of an organozinc compound with an organic halide over a nickel or palladium based homogeneous or heterogeneous catalyst,141 and pinacol coupling, which is an acid catalyzed homo coupling of a carbonyl compound to form a 1,2-diol.142 Though the reactions over metal doped mesoporous supports have not yet been reported, extensive research is ongoing in an effort to design suitable mesoporous catalysts for carrying out these coupling reactions under mild conditions.
The mesoporous Cu–Fe-hydrotalcite solid catalyst is a ligand free, novel, environmentally green catalyst for C–S coupling reactions using thiols with different aryl halides in DMF solvent and with K2CO3 base. The homogeneous mixed phase of two oxides (CuO and Fe2O3), good BET surface area (123 m2 g−1) and large pore diameter (∼15 nm) mainly contribute to the observed high conversion of products achieved within a reaction time of 4–10 h.143 CuO well-dispersed on mesoporous silica prepared by a sol–gel route is a highly reusable heterogeneous support for S-arylation of aryl iodides with thiols in the presence of Cs2CO3 base and DMSO solvent. Cu(Oac)2 loaded on organic inorganic hybrid mesoporous silica functionalized with furfural groups is also applied for aryl-sulfur coupling of thiophenol with aryl iodide in the presence of K2CO3 base and DMF solvent.144 The yield observed for different substituted diaryl sulfides was very satisfactory with proper reusable efficiency shown up to the 5th cycle. A synthesis scheme of mesoporous Cu grafted furfural functionalized organic–inorganic hybrid silica is shown in Fig. 21. A probable mechanism has also been suggested showing generation of Cu(0) active species from Cu(II) grafted organosilica in the presence of a base, followed by the consecutive oxidation addition of aryl iodide and thiophenol to form another intermediate. As observed in the catalytic cycle the intermediate formed releases an aryl-sulfur coupled product with regeneration of the active species via a reductive elimination step (Fig. 21).144
 | ||
| Fig. 21 Synthesis scheme of mesoporous Cu grafted furfural functionalized organosilica and its role in the mechanistic catalytic C–S cross coupling reaction cycle. | ||
To avoid the toxicity and hazards of the polar solvents used in different C–S coupling reactions, we explored the reaction in a completely green solvent, water, over NiO–ZrO2 self-assembled mesoporous nanocrystals at a relatively lower temperature (353 K).145 4-Chlorothiophenol is used as a sulfur source and different substituted aryl iodides are used to produce value added aryl-sulfur compounds with an appreciable yield. NiO species present in the material mainly take the active part in the catalysis which is illustrated with a proposed mechanism in Fig. 20. As an alternative to volatile and toxic thiol or foul-smelling thiophenol sulfur sources, which lead to enormous environmental and safety problems, thiourea is widely used nowadays for large scale operations. Three component one-pot coupling of aryl halides, benzyl bromides and thiourea in aqueous media is another significant approach towards green organic synthesis.146 A Cu-grafted mesoporous organo functionalized SBA-15 catalyst synthesized via a simple post-grafting technique was employed for this one-pot thioetherification carried out at a temperature of 373 K. A significant yield of aryl sulfides is obtained using this heterogeneous, non-toxic Cu-mesoporous catalyst.146
There are also other coupling reactions like C–N and C–O, etc. which to the best of our knowledge have not yet been observed to proceed over heterogeneous mesoporous materials.
Another highly significant three component coupling reaction is A3 coupling, i.e. the coupling of aldehyde, alkyne and amine, used extensively in the manufacturing of various nitrogen-containing heterocycles, natural products and biologically active compounds, etc. to form propargylamine in a convenient approach. Though many metals viz. Au, Ag, Cu, In, etc. based homogeneous catalysts showed efficiency for A3 coupling reactions,148 but due to some disadvantages for e.g. difficulty of separation of those catalysts from reaction mixture, lack of recycling efficiency and the possibility of metal leaching etc. metal immobilized heterogeneous supports are much more desirable for such reactions.149 Ag nanoparticle supported mesoporous SBA-15 has been proven to be highly efficient for A3 coupling reactions in glycol as a ‘green’ solvent at a temperature of 373 K. The product conversion observed was believed to be highly dependent on Ag particle size and a high yield of propargylamines was obtained with ca. 8 nm optimized size Ag-loaded SBA-15.150 A 2D-hexagonal periodic mesoporous organosilica containing imidazolium ionic liquid framework synthesized in acidic conditions with P123 surfactant has been functionalized with 0.2 mol% of Au and a thorough investigation was performed to study the catalytic ability of that Au loaded PMO heterogeneous support for the A3 coupling reaction under mild conditions.151 In Fig. 22 we have illustrated the synthesis of the Au@ionic liquid PMO catalyst and its application in an A3 coupling reaction. Chloroform has been proven to be the most appropriate solvent for the reaction at a relatively lower temperature (333 K) with a high average yield of around 70–80% for the corresponding propargylamine derivatives.
 | ||
| Fig. 22 A synthesis scheme for mesoporous Au nanoparticles doped imidazole functionalized PMO and an A3 coupling reaction over this catalyst. | ||
Biginelli condensation is another three component one-pot reaction which creates 3,4-dihydropyrimidin-2(1H)-ones from aryl aldehyde, ethyl acetoacetate (EAA) and urea in the presence of a Brønsted or Lewis acid as a catalyst. In 2010, an Al–MCM-41 mesoporous catalyst was employed for this reaction in octane solvent to obtain various essential substituted dihydropyrimidinones with high yields and a high catalytic yield was obtained with a small loss of activity after repeated use.152 Later in 2012, superparamagnetic Fe3O4 nanoparticles impregnated on cysteine functionalized mesoporous SBA-15 was designed and it has been proven to be a highly reusable and magnetically recoverable catalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones via the Biginelli reaction in pollution free ethanol solvent.153 A method of synthesis of the Fe3O4@cysteine functionalized SBA-15 material is shown in Fig. 23. An idea of a one-pot reaction and separation of the catalyst with a large bar magnet is also illustrated in this figure. The catalyst allowed for a high yield of products using different substituted aldehydes within a very short reaction time and a suitable catalytic cycle was suggested to be taking place over the Lewis acidic Fe3O4 active sites of the catalyst. A more environment friendly approach was made by Pramanik et al. who synthesized a novel phosphonic acid functionalized 2D hexagonal mesoporous organosilica and applied this metal free catalyst for a Biginelli condensation reaction under solvent-free conditions at 333 K.154 The reaction pathway is very clean with a very high yield of the products obtained and good recycling efficiency. The high activity of this heterogeneous organocatalyst can be attributed to the high Brønsted acidity of the phosphonic group along with the huge surface area and large pore diameter of the silica support.
 | ||
| Fig. 23 Magnetically recoverable Fe3O4@cysteine grafted mesoporous SBA-15 catalyst and its role in a one-pot Biginelli condensation reaction. | ||
Besides the above mentioned organic reactions taking place over mesoporous supports, other reactions like Diels–Alder reactions, hydroformylation of olefins, aldehydes, polymerization reactions etc. are also found to be efficiently catalyzed by some metal supported or metal-free mesoporous materials under mild reaction conditions.155–157 Further, hierarchical porosity can be introduced in the ordered porous silica composites by using anionic polystyrene spheres and triblock copolymers as templates158 and the resulting materials can potentially be utilized as supports in heterogeneous catalysis for bulkier molecules where diffusion of the reactant molecules could be facilitated.
5. Future perspectives and conclusions
To give an overview of the potential of mesoporous siliceous and non-siliceous materials in liquid phase heterogeneous catalysis, the present review offers a concise idea showing numerous examples of organic transformations with proper illustrations. Additionally, an outline of the synthesis strategies of the functionalized mesoporous materials presented here helps with suggestive information about how to design and prepare the mesoporous catalysts, tune their pore structure and specific surface area as well as how to impregnate them with various active transition metal nanoparticles, complexes or organic functional groups at the pore surface. Based on this one can prepare a number of new metal doped and organically functionalized mesoporous materials and explore their potential in versatile catalytic transformations under liquid phase reaction conditions. Replacing conventional hazardous, toxic homogeneous catalysts, which create serious environmental problems and ecological imbalance, many reactions mediated by those metallocatalysts or metal-free heterogeneous organocatalysts are carried out in aqueous solvents or without solvent, at lower temperatures or without the use of any toxic reagents. These green catalytic pathways are in high demand for the development of industrial manufacturing processes. Thus, it is expected that in future, more and more applications of these green catalysts in each and every industrial fine chemical synthesis will create a pollution free world for the benefit of all human beings.List of abbreviations
| nm | Nanometer |
| 3D | 3 dimensional |
| CMC | Critical micelle concentration |
| $ | Dollar sign |
| VO(acac)2 | Vanadyl(IV) acetylacetonate |
| Cp* | Cyclopentadienyl |
| MPa | Megapascal |
| RT | Room temperature |
| HMS | Hexagonal mesoporous silica |
| EA | Ethyl acrylate |
| ECA | Ethyl cyanoacetate |
| EclA | Ethyl chloroacetate |
| EAA | Ethyl acetoacetate |
| TMG | 1,1,3,3-Tetramethylguanidinium |
| NP | Nanoparticle |
| BET | Brunauer–Emmett–Teller |
| DMF | Dimethyl formamide |
| DMSO | Dimethyl sulfoxide |
| AIBN | 2,2′-Azobis(isobutyronitrile) |
Acknowledgements
AB wishes to thank DST, New Delhi for financial support through DST-SERB and DST-UKIERI research grants. NP is grateful to Department of Atomic Energy, Government of India for financial support.Notes and references
- M. H. Valkenberg and W. F. Hölderich, Catal. Rev., 2002, 44, 321 CAS.
- B. Lindström and L. J. Pettersson, A brief history of catalysis, CATTECH, 2003, 7, 4130 Search PubMed.
- J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- N. Pal and A. Bhaumik, Adv. Colloid Interface Sci., 2013, 189–190, 21 CrossRef CAS PubMed.
- S. Lopez-Orozco, A. Inayat, A. Schwab, T. Selvam and W. Schwieger, Adv. Mater., 2011, 23, 2602 CrossRef CAS PubMed.
- Z. M. Li, Y. Zhou, D. J. Tao, W. Huang, X. S. Chen and Z. Yang, RSC Adv., 2014, 4, 12160 RSC.
- P. B. Venuto, Microporous Mater., 1994, 2, 297 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, C. E. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS PubMed.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1 CrossRef CAS PubMed.
- C. Perego and R. Millini, Chem. Soc. Rev., 2013, 42, 3956 RSC.
- R. M. Martín-Aranda and J. Cčjka, Top. Catal., 2010, 53, 141 CrossRef.
- S. Fujita and S. Inagaki, Chem. Mater., 2008, 20, 891–908 CrossRef CAS.
- J. Rouquerol, D. Avnir, C. W. Fairbridge, D. H. Everett, J. M. Haynes, N. Pernicone, J. D. F. Ramsay, K. S. W. Sing and K. K. Unger, Pure Appl. Chem., 1994, 66, 1739 CrossRef CAS.
- D. Sengupta, J. Saha, G. De and B. Basu, J. Mater. Chem. A, 2014, 2, 3986 CAS.
- C. M. A. Parlett, K. Wilson and A. F. Lee, Chem. Soc. Rev., 2013, 42, 3876 RSC.
- T. Yokoi, M. Yoshioka, H. Ima and T. Tatsumi, Angew. Chem., Int. Ed., 2009, 48, 9884–9887 CrossRef CAS PubMed.
- M. Pramanik and A. Bhaumik, Chem.–Eur. J., 2013, 19, 8507 CrossRef CAS PubMed.
- N. Pal, M. Paul and A. Bhaumik, Appl. Catal., A, 2011, 393, 153 CrossRef CAS PubMed.
- N. Pal, M. Paul and A. Bhaumik, J. Solid State Chem., 2011, 184, 1805 CrossRef CAS PubMed.
- T. Yanagisawa, T. Shimizu, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn., 1990, 63, 988 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- U. Ciesla and F. Schüth, Microporous Mesoporous Mater., 1999, 27, 131 CrossRef CAS.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS.
- X. Lai, X. Li, W. Geng, J. Tu, J. Li and S. Qiu, Angew. Chem., Int. Ed., 2007, 46, 738 CrossRef CAS PubMed.
- C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696 CrossRef CAS PubMed.
- X. P. Fang, X. Q. Yu, S. F. Liao, Y. F. Shi, Y. S. Hu, Z. X. Wang, G. D. Stucky and L. Q. Chen, Microporous Mesoporous Mater., 2012, 151, 418–423 CrossRef CAS PubMed.
- A. Bhaumik and S. Inagaki, J. Am. Chem. Soc., 2001, 123, 691–696 CrossRef CAS PubMed.
- J. Liu, T. Y. Yang, D. W. Wang, G. Q. M. Lu, D. Y. Zhao and S. Z. Qiao, Nat. Commun., 2013, 4, 2798 Search PubMed.
- K. Ariga, A. Vinu, Y. Yamauchi, Q. M. Ji and J. P. Hill, Bull. Chem. Soc. Jpn., 2012, 85, 1–32 CrossRef CAS.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. M. Zhang, Z. H. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed.
- A. J. B. Robertson, Platinum Met. Rev., 1975, 19, 64 CAS.
- C. Elschenbroich, Organometallics, Wiley-VCH, Weinheim, 2006, ISBN 978-3-527-29390-2 Search PubMed.
- W. K. Lewis and E. D. Ries, Ind. Eng. Chem., 1925, 17, 593 CrossRef CAS.
- X. S. Zhao, X. Y. Bao, W. Guo and F. Y. Lee, Mater. Today, 2006, 9, 32 CrossRef CAS.
- K. Wilson and J. H. Clark, Pure Appl. Chem., 2000, 72, 1313 CrossRef CAS.
- S. Samanta, N. K. Mal and A. Bhaumik, J. Mol. Catal. A: Chem., 2005, 236, 7 CrossRef CAS PubMed.
- N. Pal, E.-B. Cho and D. Kim, RSC Adv., 2014, 4, 9213 RSC.
- A. Bhaumik and T. Tatsumi, J. Catal., 2000, 189, 31 CrossRef CAS.
- Y. Zhang, F. Gao, H. Wan, C. Wu, Y. Kong, X. Wu, B. Zhao, L. Dong and Y. Chen, Microporous Mesoporous Mater., 2008, 113, 393 CrossRef CAS PubMed.
- N. Pal, E.-B. Cho, D. Kim and M. Jaroniec, J. Phys. Chem. C, 2014, 118, 15892 CAS.
- W. Huang, J. N. Kuhn, C.-K. Tsung, Y. Zhang, S. E. Habas, P. Yang and G. A. Somorjai, Nano Lett., 2008, 8, 2027 CrossRef CAS PubMed.
- S. Verma, M. Nandi, A. Modak, S. L. Jain and A. Bhaumik, Adv. Synth. Catal., 2011, 353, 1897–1902 CrossRef CAS.
- Y. Rao and D. M. Antonelli, J. Mater. Chem., 2009, 19, 1937 RSC.
- A. Modak, M. Nandi and A. Bhaumik, Catal. Today, 2012, 198, 45 CrossRef CAS PubMed.
- S. Jana, B. Dutta, R. Bera and S. Koner, Langmuir, 2007, 23, 2492 CrossRef CAS PubMed.
- E. A. Prasetyanto and S.-E. Park, Bull. Korean Chem. Soc., 2008, 29, 1033 CrossRef CAS.
- P. Selvam, V. M. Ravat and V. Krishna, J. Phys. Chem. C, 2011, 115, 1922 CAS.
- W. Tanglumlert, T. Imae, T. J. White and S. Wongkasemjit, Catal. Commun., 2009, 10, 1070 CrossRef CAS PubMed.
- P. Wu, Z. Xiong, K. P. Loh and X. S. Zhao, Catal. Sci. Technol., 2011, 1, 285 CAS.
- Z. Bohström, I. Rico-Lattes and K. Holmberg, Green Chem., 2010, 12, 1861 RSC.
- A. K. Patra, A. Dutta and A. Bhaumik, Chem.–Eur. J., 2013, 19, 12388 CrossRef CAS.
- U. Mandi, M. Pramanik, A. S. Roy, N. Salam, A. Bhaumik and S. M. Islam, RSC Adv., 2014, 4, 15431 RSC.
- P. Zhang, H. Li, G. M. Veith and S. Dai, Adv. Mater., 2015, 27, 234 CrossRef CAS PubMed.
- P. Zhang, Y. Gong, H. Li, Z. Chen and Y. Wang, Nat. Commun., 2013, 4, 1593 CrossRef PubMed.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed., 2010, 49, 3356 CrossRef CAS PubMed.
- P. Zhang, H. Li and Y. Wang, Chem. Commun., 2014, 50, 6312 RSC.
- P. Zhang, Y. Wang, J. Yao, C. Wang, C. Yan, M. Antonietti and H. Li, Adv. Synth. Catal., 2011, 353, 1447 CrossRef CAS.
- S. Martínez-González, A. Gómez-Avilés, O. Martynyuk, A. Pestryakov, N. Bogdanchikova and V. Cortés Corberán, Catal. Today, 2014, 227, 65 CrossRef PubMed.
- I. Tamiolakis, I. N. Lykakis, A. P. Katsoulidis, M. Stratakis and G. S. Armatas, Chem. Mater., 2011, 23, 4204 CrossRef CAS.
- L. Geng, X. Zhang, W. Zhang, M. Jia and G. Liu, Chem. Commun., 2014, 50, 2965 RSC.
- Z.-A. Qiao, P. Zhang, S.-H. Chai, M. Chi, G. M. Veith, N. C. Gallego, M. Kidder and S. Dai, J. Am. Chem. Soc., 2014, 136, 11260 CrossRef CAS PubMed.
- N. Salam, P. Mondal, J. Mondal, A. S. Roy, A. Bhaumik and S. M. Islam, RSC Adv., 2012, 2, 6464 RSC.
- D. H. Koo, M. Kim and S. Chang, Org. Lett., 2005, 7, 5015 CrossRef CAS PubMed.
- V. Ayala, A. Corma, M. Iglesias and F. Sánchez, J. Mol. Catal. A: Chem., 2004, 221, 201 CAS.
- P. Zhang, Y. Wang, H. Li and M. Antonietti, Green Chem., 2012, 14, 1904 RSC.
- C.-F. Chang and S.-T. Liu, J. Mol. Catal. A: Chem., 2009, 299, 121 CrossRef CAS PubMed.
- S. Gontier and A. Tuel, J. Catal., 1995, 157, 124 CrossRef CAS.
- J. Evans, A. B. Zaki, M. Y. El-Sheikh and S. A. El-Safty, J. Phys. Chem. B, 2000, 104, 10271 CrossRef CAS.
- C. Perego, A. Carati, P. Ingallina, M. A. Mantegazza and G. Bellussi, Appl. Catal., A, 2001, 221, 63 CrossRef CAS.
- A. Bhaumik, M. P. Kapoor and S. Inagaki, Chem. Commun., 2003, 470 RSC.
- X. Liang, R. Yang, G. Li and C. Hu, Microporous Mesoporous Mater., 2013, 182, 62 CrossRef CAS PubMed.
- A. Dubey, M. Choi and R. Ryoo, Green Chem., 2006, 8, 144 RSC.
- P. Borah, X. Ma, K. T. Nguyen and Y. Zhao, Angew. Chem., Int. Ed., 2012, 51, 7756 CrossRef CAS PubMed.
- P. Zhang, Y. Gong, H. Li, Z. Chen and Y. Wang, RSC Adv., 2013, 3, 5121 RSC.
- P. Sreenivasulu, D. Nandan, M. Kumar and N. Viswanadham, J. Mater. Chem. A, 2013, 1, 3268 CAS.
- E. Rodríguez-Castellón, L. Díaz, P. Braos-García, J. Mérida-Robles, P. Maireles-Torres, A. Jiménez-López and A. Vaccari, Appl. Catal., A, 2003, 240, 83 CrossRef.
- G. Liu, J. Wang, T. Huang, X. Liang, Y. Zhang and H. Li, J. Mater. Chem., 2010, 20, 1970 RSC.
- R. Liu, T. Cheng, L. Kong, C. Chen, G. Liu and H. Li, J. Catal., 2013, 307, 55 CrossRef CAS PubMed.
- J. Liu, S. Zou, L. Xiao and J. Fan, Catal. Sci. Technol., 2014, 4, 441 CAS.
- M. Paul, N. Pal and A. Bhaumik, Eur. J. Inorg. Chem., 2010, 5129 CrossRef CAS.
- V. N. Sapunov, M. Y. Grigoryev, E. M. Sulman, M. B. Konyaeva and V. G. Matveeva, J. Phys. Chem. A, 2013, 117, 4073 CrossRef CAS PubMed.
- Z. Li, J. Liu, C. Xia and F. Li, ACS Catal., 2013, 3, 2440 CrossRef CAS.
- M. Nandi, J. Mondal, K. Sarkar, Y. Yamauchi and A. Bhaumik, Chem. Commun., 2011, 47, 6677 RSC.
- M. Pramanik, M. Nandi, H. Uyama and A. Bhaumik, Green Chem., 2012, 14, 2273 RSC.
- S. K. Das, M. K. Bhunia, A. K. Sinha and A. Bhaumik, ACS Catal., 2011, 1, 493 CrossRef CAS.
- T. Tsuchimoto, K. Tobita, T. Hiyama and S.-I. Fukuzawa, J. Org. Chem., 1997, 62, 6997 CrossRef CAS.
- X. Zhang, T. Lin, R. Li, T. Bai and G. Zhang, Ind. Eng. Chem. Res., 2012, 51, 3541 CrossRef CAS.
- S. Selvakumar and A. P. Singh, Catal. Lett., 2009, 128, 363 CrossRef CAS PubMed.
- Y. Rao, M. Trudeau and D. Antonelli, J. Am. Chem. Soc., 2006, 128, 13996 CrossRef CAS PubMed.
- J. Dou and H. C. Zeng, J. Phys. Chem. C, 2012, 116, 7767 CAS.
- C. Anand, B. Sathyaseelan, L. Samie, A. Beitollahi, R. Pradeep Kumar, M. Palanichamy, V. Murugesan, E.-R. Kenawy, S. S. Al-Deyab and A. Vinu, Microporous Mesoporous Mater., 2010, 134, 87 CrossRef CAS PubMed.
- A. R. Silva, L. Carneiro, A. P. Carvalho and J. Pires, Catal. Sci. Technol., 2013, 3, 2415 CAS.
- Q. Zhao, Q. Wang, D. Wu, X. Fu, T. Jiang and H. Yin, J. Ind. Eng. Chem., 2012, 18, 1326 CrossRef CAS PubMed.
- A. K. Singh, S. D. Fernando and R. Hernandez, Energy Fuels, 2007, 21, 1161 CrossRef CAS.
- Q. Yang, J. Liu, L. Zhang and C. Li, J. Mater. Chem., 2009, 19, 1945 RSC.
- A. Sakthivel, K. Komura and Y. Sugi, Ind. Eng. Chem. Res., 2008, 47, 2538 CrossRef CAS.
- V. V. Guerrero and D. F. Shantz, Ind. Eng. Chem. Res., 2009, 48, 10375 CrossRef CAS.
- L. Xu, Y. Wang, X. Yang, X. Yu, Y. Guo and J. H. Clark, Green Chem., 2008, 10, 746 RSC.
- E. Li and V. Rudolph, Energy Fuels, 2008, 22, 145 CrossRef CAS.
- A. Sinhamahapatra, N. Sutradhar, M. Ghosh, H. C. Bajaj and A. B. Panda, Appl. Catal., A, 2011, 402, 87 CrossRef CAS PubMed.
- S. B. Umbarkar, T. V. Kotbagi, A. V. Biradar, R. Pasricha, J. Chanale, M. K. Dongare, A.-S. Mamede, C. Lancelot and E. Payen, J. Mol. Catal. A: Chem., 2009, 310, 150 CrossRef CAS PubMed.
- M. Iwamoto, Y. Tanaka, N. Sawamur and S. Namba, J. Am. Chem. Soc., 2003, 125, 13032 CrossRef CAS PubMed.
- M. S. Khayoon and B. H. Hameed, Appl. Catal., A, 2013, 464–465, 191 CrossRef CAS PubMed.
- M. Rat, M. H. Zahedi-Niaki, S. Kaliaguine and T. O. Do, Microporous Mesoporous Mater., 2008, 112, 26 CrossRef CAS PubMed.
- C. Noda Pérez, C. A. Pérez, C. A. Henriques and J. L. F. Monteiro, Appl. Catal., A, 2004, 272, 229 CrossRef PubMed.
- L. Faba, E. Díaz and S. Ordóñez, ChemSusChem, 2013, 6, 463 CrossRef CAS PubMed.
- P. Bai, P. Wu, Z. Yan and X. S. Zhao, J. Mater. Chem., 2009, 19, 1554 RSC.
- G. D. Yadav and P. Aduri, J. Mol. Catal. A: Chem., 2012, 355, 142 CrossRef CAS PubMed.
- E. G. Doyagüez, F. Calderón, F. Sánchez and A. Fernández-Mayoralas, J. Org. Chem., 2007, 72, 9353 CrossRef PubMed.
- G. Postole, B. Chowdhury, B. Karmakar, K. Pinki, J. Banerji and A. Auroux, J. Catal., 2010, 269, 110 CrossRef CAS PubMed.
- M. N. Pahalagedara, L. R. Pahalagedara, C.-H. Kuo, S. Dharmarathna and S. L. Suib, Langmuir, 2014, 30, 8228 CrossRef CAS PubMed.
- K. M. Parida, S. Mallick, P. C. Sahoo and S. K. Rana, Appl. Catal., A, 2010, 381, 226 CrossRef CAS PubMed.
- K.-S. Kim, J. H. Song, J.-H. Kim and G. Seo, Stud. Surf. Sci. Catal., 2003, 146, 505 CrossRef CAS.
- R. Zhu, J. Shen, Y. Wei and F. Zhang, New J. Chem., 2011, 35, 1861 RSC.
- A. Baeyer and V. Villiger, Chem. Ber., 1899, 32, 3625 CrossRef.
- M. Paul, N. Pal, J. Mondal, M. Sasidharan and A. Bhaumik, Chem. Eng. Sci., 2012, 71, 564 CrossRef CAS PubMed.
- E.-Y. Jeong, M. B. Ansari and S.-E. Park, ACS Catal., 2011, 1, 855 CrossRef CAS.
- A. Sinhamahapatra, A. Sinha, S. K. Pahari, N. Sutradhar, H. C. Bajaja and A. B. Panda, Catal. Sci. Technol., 2012, 2, 2375 CAS.
- R. Palkovits, C.-M. Yang, S. Olejnik and F. Schüth, J. Catal., 2006, 243, 93 CrossRef CAS PubMed.
- C. Ngamcharussrivichai, P. Wu and T. Tatsumi, J. Catal., 2004, 227, 448 CrossRef CAS PubMed.
- X. Wang, C.-C. Chen, S.-Y. Chen, Y. Mou and S. Cheng, Appl. Catal., A, 2005, 281, 47 CrossRef CAS PubMed.
- A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed.
- A. Modak, J. Mondal and A. Bhaumik, Green Chem., 2012, 14, 2840 RSC.
- A. Modak, J. Mondal, M. Sasidharan and A. Bhaumik, Green Chem., 2011, 13, 1317 RSC.
- V. L. Budarin, J. H. Clark, R. Luque, D. J. Macquarrie and R. J. White, Green Chem., 2008, 10, 382 RSC.
- K. R. Reddy, M. Suresh, M. L. Kantam, S. K. Bhargava and P. Srinivasu, Ind. Eng. Chem. Res., 2014, 53, 18630 CrossRef CAS.
- K. Sarkar, M. Nandi, M. Islam, M. Mubarak and A. Bhaumik, Appl. Catal., A, 2009, 352, 81 CrossRef CAS PubMed.
- P. Han, H. Zhang, X. Qiu, X. Ji and L. Gao, J. Mol. Catal. A: Chem., 2008, 295, 57 CrossRef CAS PubMed.
- H. R. Choi, H. Woo, S. Jang, J. Y. Cheon, C. Kim, J. Park, K. H. Park and S. H. Joo, ChemCatChem, 2012, 4, 1587 CrossRef CAS.
- B. Karimi, D. Elhamifar, J. H. Clark and A. J. Hunt, Chem.–Eur. J., 2010, 16, 8047 CrossRef CAS PubMed.
- K. M. Parida, S. Singha, P. C. Sahoo and S. Sahu, J. Mol. Catal. A: Chem., 2011, 342–343, 11 CrossRef CAS PubMed.
- J. Mondal, A. Modak and A. Bhaumik, J. Mol. Catal. A: Chem., 2011, 350, 40 CrossRef CAS PubMed.
- X. Ma, Y. Zhou, J. Zhang, A. Zhu, T. Jiang and B. Han, Green Chem., 2008, 10, 59 RSC.
- B. Baruwati, D. Guin and S. V. Manorama, Org. Lett., 2007, 9, 5377 CrossRef CAS PubMed.
- S.-H. Huang, C.-H. Liu and C.-M. Yang, Green Chem., 2014, 16, 2706 RSC.
- H. Li, J. Chen, Y. Wan, W. Chai, F. Zhang and Y. Lu, Green Chem., 2007, 9, 273 RSC.
- Y. Wan, H. Wang, Q. Zhao, M. Klingstedt, O. Terasaki and D. Zhao, J. Am. Chem. Soc., 2009, 131, 4541 CrossRef CAS PubMed.
- B. Karimi and F. K. Esfahani, Chem. Commun., 2011, 47, 10452 RSC.
- A. P. Singh and P. Sharma, RSC Adv., 2014, 4, 43070 RSC.
- S. W. Smith and G. C. Fu, Angew. Chem., 2008, 120, 9474 CrossRef.
- Y.-S. Yang, Z.-L. Shen and T.-P. Loh, Org. Lett., 2009, 11, 2213 CrossRef CAS PubMed.
- D. K. Dumbrea, P. R. Selvakannana, S. K. Patil, V. R. Choudhary and S. K. Bhargava, Appl. Catal., A, 2014, 476, 54 CrossRef PubMed.
- J. Mondal, A. Modak, A. Dutta and A. Bhaumik, Dalton Trans., 2011, 5228 RSC.
- N. Pal and A. Bhaumik, Dalton Trans., 2012, 9161 RSC.
- J. Mondal, A. Modak, A. Dutta, S. Basu, S. N. Jha, D. Bhattacharyya and A. Bhaumik, Chem. Commun., 2012, 48, 8000 RSC.
- C.-K. Chen, Y.-W. Chen, C.-H. Lin, H.-P. Lin and C.-F. Lee, Chem. Commun., 2010, 46, 282 RSC.
- V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790 RSC.
- M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 3093 CrossRef CAS.
- G.-P. Yonga, D. Tiana, H.-W. Tonga and S.-M. Liu, J. Mol. Catal. A: Chem., 2010, 323, 40 CrossRef PubMed.
- B. Karimi, M. Gholinejad and M. Khorasani, Chem. Commun., 2012, 48, 8961 RSC.
- H. Murata, H. Ishitani and M. Iwamoto, Org. Biomol. Chem., 2010, 8, 1202 CAS.
- J. Mondal, T. Sen and A. Bhaumik, Dalton Trans., 2012, 6173 RSC.
- M. Pramanik and A. Bhaumik, ACS Appl. Mater. Interfaces, 2014, 6, 933 CAS.
- S. Minakata, T. Nagamachi, K. Nakayama, T. Suzuki and T. Tanaka, Chem. Commun., 2011, 47, 6338 RSC.
- W. Zhou and D. He, Green Chem., 2009, 11, 1146 RSC.
- M. Pramanik, M. Nandi, H. Uyama and A. Bhaumik, Catal. Sci. Technol., 2012, 2, 613 CAS.
- T. Sen, G. J. T. Tiddy, J. L. Casci and M. W. Anderson, Chem. Mater., 2004, 16, 2044 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |