CuNi/Co composites prepared by electroless deposition: structure and catalytic activity for the oxidation of cyclohexene with oxygen†
Abstract
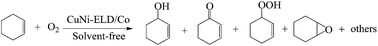
CuNi/Co composites with various Cu contents have been prepared by electroless deposition (ELD) of Cu on Co powers and characterized with ICP-OES, N2 adsorption/desorption, XRD, SEM, TEM and XPS. Their catalytic performance was examined for the allylic oxidation of cyclohexene with oxygen under solvent-free conditions. The phase structure of the Cu deposit in the composites is transformed from an amorphous phase to a crystalline phase with the increase in Cu content. The composition and microstructure of the Cu deposit in the composite significantly affect its catalytic activity for the oxidation of cyclohexene. CuNi-ELD/Co-5 composite with 6.0 wt% Cu content and amorphous Cu deposit showed the highest catalytic performance with a 46.3% conversion of cyclohexene and 88.1% total selectivity to 2-cyclohexene-1-ol (Cy-ol), 2-cyclohexene-1-one (Cy-one), 2-cyclohexene-1-hydroperoxide (Cy-HP) and cyclohexene oxide (Cy-oxide).

 Please wait while we load your content...
Please wait while we load your content...