Sol–gel nanocasting synthesis of kesterite Cu2ZnSnS4 nanorods†
Abstract
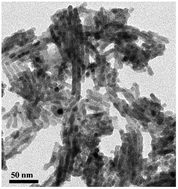
Nanocasting synthesis of quaternary chalcogenide Cu2ZnSnS4 (CZTS) nanocrystals remains a big challenge due to the difficulty in the impregnation of the quaternary precursors and the competition of the formation of binary and ternary sulfides. We herein report the first successful nanocasting synthesis of nanorods of the quaternary sulfide compound, CZTS, based on mesoporous SBA-15 template through a sol–gel process. Kesterite CZTS nanorods with a diameter of 6.8 nm and a surface area of 76.19 m2 g−1 are obtained. The compositions and concentrations of the quaternary precursors are investigated for the impregnation and crystallization of CZTS in the silica template.

 Please wait while we load your content...
Please wait while we load your content...