Water mediated reactions: TiO2 and ZnO nanoparticle catalyzed multi component domino reaction in the synthesis of tetrahydroacridinediones, acridindiones, xanthenones and xanthenes†
Abstract
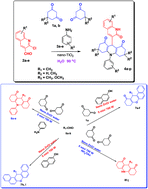
Eco-accommodation of TiO2 nanorods in the four component Domino reaction for the framing of 9-(2-oxo-1,2-dihydroquinolin-3-yl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8-(2H,5H,9H,10H)-diones 4 from 1,3-cyclohexanedione and/or dimedone 1, 2-chloro-3-formylquinoline 2 and anilines 3 in water at 90 °C are accounted for. The present methodology offers a domino reaction strategy, high yield, simple operation, recyclability and is eco-friendly. In addition, a productive, highly chemoselective ZnO catalyzed, water mediated, microwave aided synthesis of functionalized xanthenes and xanthenones 7 and acridinediones 8 in excellent yields is reported through an environmentally benevolent strategy.

 Please wait while we load your content...
Please wait while we load your content...