Mild catalytic depolymerization of low rank coals: a novel way to increase tar yield
Abstract
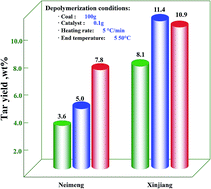
Mild catalytic depolymerization of two kinds of low-rank coals named as Neimeng and Xinjiang coals sprayed with Mo- and Fe-based catalysts were performed in a batch reactor. The obtained tar was analyzed by gas chromatography/mass spectrometry (GC/MS), and the coal and char were characterized by Raman and Fourier transform infrared spectroscopy (FTIR). The results indicated that the tar yields for Neimeng coal were increased from 3.6% to 5.0% and 7.8% while those for Xinjiang coal were increased from 8.1% to 11.4% and 10.9% after the addition of Mo- and Fe-based catalysts, respectively. Based on the GC/MS analysis, it is found that the hydroxybenzene content was significantly decreased while naphthalene content was increased in the tar derived from Neimeng coal when the Mo-based catalyst was used. In contrast, the production of light aromatics was enhanced by the addition of Fe-based catalyst. For comparison, the production of aromatics from Xinjiang coal was remarkably inhibited but the content of aliphatic hydrocarbon was significantly increased after the addition of the catalyst. The Raman and FTIR analyses results indicated that the catalyst could improve the reaction of hydrogen free radicals with char, which was beneficial to the depolymerization of coal.

 Please wait while we load your content...
Please wait while we load your content...