A novel coumarin based molecular switch for dual sensing of Zn(II) and Cu(II)†
Deblina Sarkar,
Ajoy Kumar Pramanik and
Tapan Kumar Mondal*
Department of Chemistry, Jadavpur University, Kolkata-700032, India. E-mail: tkmondal@chemistry.jdvu.ac.in
First published on 22nd December 2014
Abstract
An efficient coumarin based molecular switch for the dual sensing of two environmentally as well as biologically important cations Zn2+ and Cu2+ has been synthesized. The receptor H2L shows about 6 fold enhancement in fluorescence intensity upon the addition of Zn2+ and also exhibits quenching of emission intensity upon the addition of Cu2+ without interference of other metal ions present in solution. In the case of other metal ions no significant change in the emission intensity is observed. H2L presents a tunable system comprising of two INHIBIT logic gates with Zn2+ and Cu2+ or Zn2+ and EDTA as chemical inputs by monitoring the emission mode. An IMPLICATION logic gate is obtained with Cu2+ and EDTA as chemical inputs and emission as the output mode.
Introduction
Zinc(II) and copper(II) play a very crucial role in the human body and serve as the second and third most abundant transition metal ions after iron(III), found in the human body.1 The highest concentration of zinc in the human body is found in the brain and plays an important role in many biological processes such as regulation of gene expressions, apoptosis, neural signal transmitters and catalytic cofactors.2 However metabolic disorders of zinc(II) lead to several neurodegenerative diseases such as Parkinson's and Alzheimer's diseases.3 Again, inadequate level of zinc leads to retardation of growth, decrease in the immunological defense, eye lesion and some skin diseases.4 Copper(II) also plays a significant role in biological, environmental and chemical systems.5 The disorder in Cu(II) metabolism may lead to severe diseases, such as Alzheimer's and Wilson's diseases, amyotrophic lateral sclerosis, Menkes syndrome and haematological manifestations.6–11 Thus detection of zinc(II) and copper(II) is of utmost importance from both environmental as well as biological point of views.Development of artificial chemosensors based on fluorescence technique has emerged out to be a powerful detection tool owing to its simplicity, sensitivity and tunability.12 In few recent years, several small molecules for the detection of zinc(II),13 by the enhancement of fluorescent intensity have been reported while several reported molecules have the capability to detect copper by quenching of fluorescent intensity.14 Most of the reported chemosensors for zinc suffer the problem of interference from Cd2+ because of similarity in the electronic configuration.15 Till date only a few chemosensors with dual sensing properties for metal ions have been reported.16 Thus the development of dual chemosensors for the detection of vital elements still remains an active field of research.
Development of various chemical systems to exhibit operations such as AND, OR, NOT and their integrated operations have been carried out.17 Small molecules with more than one output channel are of utmost interest as they form the basis of molecular logic gates capable of performing several arithmetic operations.18 Various single molecules have been exploited for the construction of many useful integrated logic gates such as INHIBIT, half subtractor, half adder, full adder, and full subtractor.19 However very few IMPLICATION gates have been reported so far.20 Recently L. Zhao et al. has reported several molecular logic gates based on salicylidine Schiff base.21 In our previous work we have reported an INHIBIT logic gate based on a coumarin Schiff base with Al3+ and EDTA as chemical inputs.22
In this present work we have reported the synthesis and spectral characterizations of a coumarin based organic framework which has the dual sensing property for zinc(II) and copper(II) by subsequent enhancement and quenching of fluorescence intensity. Coumarin framework exhibits various interesting photophysical properties such as Stokes shift and visible excitation and emission wavelengths, and also has high importance as fluorescent dyes.23 Only a few coumarin based chemosensors are reported so far for the dual sensing of metal ions.24 Till date, there has been no report of coumarin based chemosensor for dual sensing of Zn2+ and Cu2+. Only a few reported chemosensors are known to selectively recognize these two vital elements zinc and copper. S. Wang et al. has reported a binaphthyl-derived salicylidene Schiff base for dual sensing of Cu(II) and Zn(II).25 Y. Liu et al. has reported a fluorescent ‘off–on–off’ probe for relay recognition of Zn2+ and Cu2+ derived from N,N-bis(2-pyridylmethyl)amine26 while L. Qua et al. has recently reported a pyridoxal-based dual chemosensor for zinc and copper.27 However, most of these reported chemosensors have lower binding constants for Zn2+ and Cu2+ and also higher limit of detection, compared to that of our newly developed receptor H2L. In certain cases, the receptor is synthesized using several steps along with the use of reagents which are difficult to handle. Whereas in the present case, the synthetic route towards H2L is very facile and economically cheap. The developed chemosensor is highly efficient in the detection of zinc(II) with enhancement of fluorescence intensity by 6 fold while it detects copper by quenching of emission intensity, by 7 folds. H2L presents a tunable system comprising of two INHIBIT logic gates with Zn2+ and Cu2+ or Zn2+ and EDTA as chemical inputs. An IMPLICATION logic gate is obtained with Cu2+ and EDTA as chemical inputs and emission as the output mode. H2L exhibits very high selectivity only for copper and zinc with no interference from any other metal ions including cadmium. Thus the synthesized chemosensor H2L is an important addition to the list of few reported simple organic molecules which can detect zinc and copper selectively.
Results and discussion
Synthesis and spectral characterisation
Synthetic route towards H2L involves a very facile and economically cheap route using Schiff base condensation of 3-acetyl-4-hydroxycoumarin with p-phenylenediamine in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in methanolic medium under refluxing condition for 6 hours (Scheme 1).
1 molar ratio in methanolic medium under refluxing condition for 6 hours (Scheme 1).
The ligand H2L may exist in equilibrium in the keto and enol form by excited state intramolecular proton transfer process (ESIPT) which is further supported by the small energy gap (ΔE = 8.025 kcal mol−1) between the two tautomeric forms (Scheme 2). The energies calculated by DFT/B3LYP/6-31G(d,p) method indicate that the keto form is more stable than the enol form by 8.025 kcal mol−1. IR spectrum of H2L taken in KBr disk shows a stretching band at 1698 cm−1 corresponding to lactone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, the keto C
O, the keto C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C appears at 1610 cm−1 and 1547 cm−1 respectively (Fig. S1†). 1H-NMR spectra are recorded in CDCl3 which shows a band at around δ 15.45 which is due to the hydrogen bonded NH proton (Fig. S4†). This peak vanishes in the H2L–Zn2+ complex indicating co-ordination to the metal centre through N donating site of the enol form (Fig. S5†). The aromatic protons in H2L appear as expected in the region δ 8.10–7.27. The –N
C appears at 1610 cm−1 and 1547 cm−1 respectively (Fig. S1†). 1H-NMR spectra are recorded in CDCl3 which shows a band at around δ 15.45 which is due to the hydrogen bonded NH proton (Fig. S4†). This peak vanishes in the H2L–Zn2+ complex indicating co-ordination to the metal centre through N donating site of the enol form (Fig. S5†). The aromatic protons in H2L appear as expected in the region δ 8.10–7.27. The –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)– protons appear at δ 2.79 as singlet. All aromatic protons appear at a bit downfield position compared to that of H2L, which can clearly be explained due to the co-ordination of Zn2+ with H2L. However, the coordination of H2L with Cu2+ could not be studied by NMR spectroscopy owing to the paramagnetic nature of Cu2+ ion. Mass spectrum shows m/z peak corresponding to H+[H2L] at 481.2 for H2L (Fig. S6†). For H2L–Zn2+ complex the strong peak at 703.3 correspond to Na[Zn2(L)Cl2]+ along with a weak peak at 739.4 corresponding to Na[Zn2(L)Cl2(H2O)2]+ species (Fig. S7†). For H2L–Cu2+ complex the strong peak at 699.2 correspond to Na[Cu2(L)Cl2]+ along with a weak peak at 717.3 corresponding to Na[Cu2(L)Cl2(H2O)]+ (Fig. S8†) species supporting 1
C(CH3)– protons appear at δ 2.79 as singlet. All aromatic protons appear at a bit downfield position compared to that of H2L, which can clearly be explained due to the co-ordination of Zn2+ with H2L. However, the coordination of H2L with Cu2+ could not be studied by NMR spectroscopy owing to the paramagnetic nature of Cu2+ ion. Mass spectrum shows m/z peak corresponding to H+[H2L] at 481.2 for H2L (Fig. S6†). For H2L–Zn2+ complex the strong peak at 703.3 correspond to Na[Zn2(L)Cl2]+ along with a weak peak at 739.4 corresponding to Na[Zn2(L)Cl2(H2O)2]+ species (Fig. S7†). For H2L–Cu2+ complex the strong peak at 699.2 correspond to Na[Cu2(L)Cl2]+ along with a weak peak at 717.3 corresponding to Na[Cu2(L)Cl2(H2O)]+ (Fig. S8†) species supporting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex formation for both zinc and copper complexes.
2 complex formation for both zinc and copper complexes.
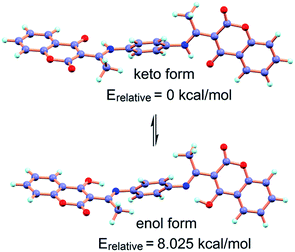 | ||
| Scheme 2 Keto–enol tautomerism of H2L and their relative energies calculated by DFT/B3LYP/6-31G(d,p) method. | ||
Cation sensing studies of H2L
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v CH3CN
1, v/v CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O using HEPES buffered solution at pH = 7.2. Gradual addition of Zn2+ (40 μM) shows a decrease in absorbance intensity at 344 nm with the appearance of a new absorption band at 265 nm (Fig. 1). Formation of this new band at 265 nm indicates the co-ordination of the receptor to Zn2+. Again, on gradual addition of Cu2+ (40 μM) formation of low energy band is observed at 459 nm along with a new absorption band at 270 nm supporting the coordination of Cu2+ to the receptor (Fig. 2). UV-vis spectrum of H2L is also studied in presence of other metals i.e. Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Co2+, Ni2+, Al3+, Cd2+ and Hg2+ but no significant changes are observed except for Hg2+ and Ni2+ (Fig. S9†).
H2O using HEPES buffered solution at pH = 7.2. Gradual addition of Zn2+ (40 μM) shows a decrease in absorbance intensity at 344 nm with the appearance of a new absorption band at 265 nm (Fig. 1). Formation of this new band at 265 nm indicates the co-ordination of the receptor to Zn2+. Again, on gradual addition of Cu2+ (40 μM) formation of low energy band is observed at 459 nm along with a new absorption band at 270 nm supporting the coordination of Cu2+ to the receptor (Fig. 2). UV-vis spectrum of H2L is also studied in presence of other metals i.e. Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Co2+, Ni2+, Al3+, Cd2+ and Hg2+ but no significant changes are observed except for Hg2+ and Ni2+ (Fig. S9†).
 | ||
Fig. 1 Change in UV-vis spectrum of H2L (20 μM) upon gradual addition of Zn2+ (40 μM) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v CH3CN 1, v/v CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. H2O. | ||
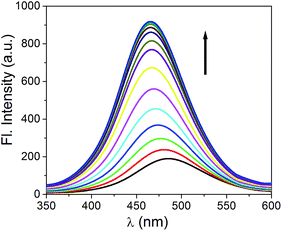 | ||
Fig. 3 Change in emission spectrum of H2L (20 μM) upon gradual addition of Zn2+ (40 μM) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v CH3CN 1, v/v CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. H2O. | ||
 | ||
Fig. 4 Change in emission spectrum of H2L (20 μM) upon gradual addition of Cu2+ (40 μM) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v CH3CN 1, v/v CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. H2O. | ||
Mole ratio plot obtained from fluorescence titration indicates that the receptor shows an increase in emission intensity till the ratio of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2L reaches ∼2, after that there is hardly any increase in emission intensity (Fig. S12†). Quenching of fluorescence intensity occurs till the ratio of Cu2+
H2L reaches ∼2, after that there is hardly any increase in emission intensity (Fig. S12†). Quenching of fluorescence intensity occurs till the ratio of Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2L reaches 2 (Fig. S13†). Jobs plot of emission intensity shows maxima in the plot corresponding to ∼0.65 mole fraction for H2L–Zn2+ complex (Fig. S14†) and at ∼0.66 mole fraction for H2L with Cu2+, reflecting 1
H2L reaches 2 (Fig. S13†). Jobs plot of emission intensity shows maxima in the plot corresponding to ∼0.65 mole fraction for H2L–Zn2+ complex (Fig. S14†) and at ∼0.66 mole fraction for H2L with Cu2+, reflecting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex formation in both the cases (Fig. S15†). From emission spectral change, limit of detection of the chemosensor for Zn2+ and Cu2+ are determined using the equation LOD = K × SD/S where ‘SD’ is the standard deviation of the blank solution and ‘S’ in the slope of the calibration curve (Fig. S16 and S17†). The limit of detection for Zn2+ is 1.94 × 10−8 M from fluorescent titration while that of Cu2+ is found to be 1.87 × 10−9 M. This result clearly demonstrates that the chemosensor is highly efficient in sensing Zn2+ as well as Cu2+ even in very minute level. From fluorescent spectral titration the association constant of H2L with Zn2+ and Cu2+ are found to be 1.8 × 109 and 1.34 × 1010 respectively (Fig. S18 and S19†).
2 complex formation in both the cases (Fig. S15†). From emission spectral change, limit of detection of the chemosensor for Zn2+ and Cu2+ are determined using the equation LOD = K × SD/S where ‘SD’ is the standard deviation of the blank solution and ‘S’ in the slope of the calibration curve (Fig. S16 and S17†). The limit of detection for Zn2+ is 1.94 × 10−8 M from fluorescent titration while that of Cu2+ is found to be 1.87 × 10−9 M. This result clearly demonstrates that the chemosensor is highly efficient in sensing Zn2+ as well as Cu2+ even in very minute level. From fluorescent spectral titration the association constant of H2L with Zn2+ and Cu2+ are found to be 1.8 × 109 and 1.34 × 1010 respectively (Fig. S18 and S19†).
Fluorescence emission intensity of H2L (20 μM) is studied in presence of other metals i.e. Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Co2+, Ni2+, Al3+, Cd2+ and Hg2+ (40 μM) in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.2) but there is hardly any change in emission intensity of H2L except in presence of Zn2+ and Cu2+ (Fig. S20†).
1, v/v, pH = 7.2) but there is hardly any change in emission intensity of H2L except in presence of Zn2+ and Cu2+ (Fig. S20†).
In order to study the selectivity of H2L for Zn2+ and Cu2+, interference experiment is carried out by recording the emission intensity of H2L (20 μM) in presence of other metal ions like Na+, K+, Ca2+, Mg2+, Mn2+, Fe3+, Cr3+, Al3+, Co2+, Ni2+, Cd2+ and Hg2+ (40 μM) before the addition of Zn2+ and Cu2+. It is observed that the various competitive metal ions do not cause any significant interference both for Cu2+ and Zn2+. The addition of Cu2+ to H2L + Zn2+ causes sharp quenching of emission intensity as expected from association constant values. Thus H2L basically shows an OFF–ON–OFF signally pattern in presence of Zn2+ and Cu2+. To verify whether Cu2+ is responsible for the quenching of emission intensity, ascorbic acid is added to mask the effect of Cu2+ and the resulting solution shows an enhancement of fluorescence intensity as expected (Fig. 5).
The effect of pH on the emission intensity of the receptor (H2L) in absence and presence of Zn2+ and Cu2+ are studied. In case of H2L there is hardly any change in fluorescence intensity in the pH range 1–4 (Fig. 6). Below pH 4 high fluorescence intensity is observed due to protonation of imine N and hydroxy O atoms preventing the excited state intramolecular proton transfer (ESIPT) process, which is responsible for the quenching of fluorescence intensity. On addition of 2 equivalents of Zn2+ the fluorescence intensity remains almost unchanged in the pH < 4, while there is a sharp increase in fluorescence intensity in the pH range 5–9 compared to H2L. But, on further increase in pH fluorescence intensity drops drastically due to the formation of Zn(OH)2 at pH > 9. On addition of Cu2+, quenching of fluorescent intensity of H2L is significant in the pH range 4–9. Thus H2L forms stable complex with Zn2+ and Cu2+ in the pH range of 5–9 hence the developed receptor H2L can detect Zn2+ and Cu2+ in this pH range. However at low pH values (pH < 4) receptor tends to combine with protons and hence becomes ineffective in detection of Zn2+ and Cu2+.
Application as logic function
The developed chemosensor H2L can be utilised as a binary logic function with dual stimulating inputs as Zn2+ and Cu2+ and emission as output. As a result of coordination of H2L with Zn2+(IN1) a new emission band appears at 466 nm. Upon gradual addition of Cu2+(IN2), the emission intensity of the band at 466 nm gets quenched. This preference of the receptor H2L in binding with Cu2+ even in presence of Zn2+ is well explained from the binding constant values. Addition of Cu2+ alone to H2L also results in quenching of emission intensity. The threshold value of fluorescence intensity is taken to be 190. OUT = 0 when intensity is less than 190; OUT = 1 when intensity is higher than 190. Now OUT = 1 only when Zn2+ is present alone. Actually it represents an AND gate with an inverter28 in one of its input. Thus the emission change at 466 nm with Zn2+ as well as Cu2+ (with an invertor) as inputs can be interpreted as a monomolecular circuit showing an INHIBIT logic function22 (Fig. 7).When EDTA (40 μM) is added to the H2L–Zn2+ complex the solution shows a decrease of emission intensity and the band at 466 nm is disappeared, suggesting that the receptor H2L has again returned to its free form. However in the absence of Zn2+, EDTA does not have any effect on the emission intensity of the receptor H2L (Fig. 8). Thus with two chemical inputs as Zn2+ and EDTA, H2L functions as an AND gate with an inverter in the EDTA input by monitoring the emission output. This function can be interpreted as a monomolecular circuit showing an INHIBIT logic function. On the other hand when EDTA is added to the H2L–Cu2+ solution, an obvious enhancement of fluorescence intensity is observed due to complex formation of EDTA with Cu2+ making the receptor free. Thus the emission intensity value at 484 nm is low only when Cu2+ is present. When Cu2+ is present along with EDTA the emission intensity at 484 nm is high. Thus it actually represents an OR with an inverter in one of its input which is also called an IMPLICATION logic gate.29
Electronic structure and sensing mechanism
To interpret the electronic structure of H2L geometry optimization has been performed by DFT/B3LYP method for keto and enol forms in singlet ground state (S0) (Scheme 2). The energy calculation in S0 state reveals that the keto form is more stable by 8.025 kcal mol−1 than the corresponding enol form which is consistent with the X-ray structure of this type of molecules.30 The geometry of H2L–Zn2+ and H2L–Cu2+ have been optimized and the energy minimized structures are shown in Fig. S21 and S22† respectively. In the complexes the chemosensor H2L binds to Zn2+ and Cu2+ through phenolic-O atom and imine-N. Contour plots of some selected molecular orbitals of H2L and its complexes with Zn2+ and Cu2+ are shown in Fig. S23–25.†To interpret the changes in electronic spectra TDDFT calculation by DFT/B3LYP/CPCM method has been carried out in acetonitrile. The intense band at 338 nm for chemosensor H2L corresponds to HOMO → LUMO transition (Table S1†). In H2L–Zn2+ the intense HOMO → LUMO transition is observed at 340 nm having ILCT character. For H2L–Cu2+ the very weak transition at 482 nm corresponds to ligand to metal charge transfer transition (LMCT) along with a strong transition at 359 nm corresponding to HOMO−1(β) → LUMO+2(β) transition has been observed.
The chemosensor H2L shows a weak emission band centered around 484 nm. Upon gradual addition of Zn2+, there is an enhancement of fluorescence intensity and a new emission band appears at 466 nm. To interprete whether the excited state intramolecular proton transfer (ESIPT)31 is responsible for the quenching of fluorescence intensity for H2L, theoretical calculations are carried out. The possible intramolecular proton transfer process in ground (S0) state has been considered (Scheme 2) and there is only 8.025 kcal mol−1 of energy difference between the two forms. So, the hydrogen transfer takes place easily resulting in quenching of fluorescence for H2L. On coordination with Zn2+ this ESIPT process is inhibited resulting in fluorescence intensity enhancement. However, the significant quenching of fluorescence intensity of H2L upon addition of Cu2+ is expected due to the paramagnetic nature of Cu2+ ion.32
Experimental
Material and methods
4-Hydroxycoumarin and 1,4-diaminobenzene were purchased from Aldrich. All other organic chemicals and inorganic salts were available from commercial suppliers and used without further purification.Elemental analysis was carried out in a 2400 Series-II CHN analyzer, Perkin Elmer, USA. HRMS mass spectra were recorded on Waters (Xevo G2 Q-TOF) mass spectrometer. Infrared spectra were taken on a RX-1 Perkin Elmer spectrophotometer with samples prepared as KBr pellets. Electronic spectral studies were performed on a Perkin Elmer Lambda 25 spectrophotometer. Luminescence property was measured using Perkin Elmer LS 55 fluorescence spectrophotometer at room temperature (298 K). NMR spectra were recorded using a Bruker (AC) 300 MHz FTNMR spectrometer in CDCl3.
The luminescence quantum yield was determined using carbazole as reference with a known ϕR of 0.42 in MeCN. The complex and the reference dye were excited at the same wavelength, maintaining nearly equal absorbance (∼0.1), and the emission spectra were recorded. The area of the emission spectrum was integrated using the software available in the instrument and the quantum yield is calculated according to the following equation:
| ϕS/ϕR = [AS/AR] × [(Abs)R/(Abs)S] × [ηS2/ηR2]. |
Synthesis of the receptor (H2L)
3-Acetyl-4-hydroxy-2H-chromen-2-one (L)33 (0.204 g, 1.0 mmol) and 1,4-diaminobenzene (0.054 g, 0.5 mmol) were refluxed for 6 hours in methanolic medium. Solvent was evaporated under reduced pressure and then dissolved in dichloromethane which is then further subjected to silica gel (60–120 mesh) column chromatographic separation. The desired light yellow solid product was obtained by elution with 35% ethyl acetate–pet-ether (v/v) mixture. Yield was, 0.399 g, 83%.Anal. calc. for C28H20N2O6 (H2L): calc. (%) C 69.99, H 4.20, N 5.83. Found (%), C 69.03, H 4.01, N 5.21. IR data (KBr, cm−1): 1698 ν(lactone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1610 ν(keto C
O); 1610 ν(keto C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1547 ν(C
O), 1547 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H NMR data (CDCl3, 300 MHz): δ 15.45 (2H, s), 8.10 (2H, d, J = 7.4 Hz), 7.60 (2H, t, J = 7.2 Hz), 7.38 (4H, s), 7.27–7.32 (4H, m), 2.79 (6H, s).
C). 1H NMR data (CDCl3, 300 MHz): δ 15.45 (2H, s), 8.10 (2H, d, J = 7.4 Hz), 7.60 (2H, t, J = 7.2 Hz), 7.38 (4H, s), 7.27–7.32 (4H, m), 2.79 (6H, s).
General method for UV-vis and fluorescence titration
Stock solution of the receptor H2L (10 μM) in [(CH3CN/H2O), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v] (at 25 °C) using HEPES buffered solution at pH = 7.2 was prepared. The solution of the guest cations using their chloride salts in the order of 100 μM were prepared in deionised water. Solutions of various concentrations containing host and increasing concentrations of cations were prepared separately. The spectra of these solutions were recorded by means of UV-vis methods. EDTA solution of 100 μM was added to the same solution where Zn2+ and Cu2+ were added gradually to H2L and emission spectra recorded. The spectra of all these solutions were also recorded by means of fluorescence methods.
1, v/v] (at 25 °C) using HEPES buffered solution at pH = 7.2 was prepared. The solution of the guest cations using their chloride salts in the order of 100 μM were prepared in deionised water. Solutions of various concentrations containing host and increasing concentrations of cations were prepared separately. The spectra of these solutions were recorded by means of UV-vis methods. EDTA solution of 100 μM was added to the same solution where Zn2+ and Cu2+ were added gradually to H2L and emission spectra recorded. The spectra of all these solutions were also recorded by means of fluorescence methods.
Job's plot by fluorescence method
A series of solutions containing H2L (10 μM), ZnCl2 and CuCl2 (10 μM) were prepared in such a manner that the sum of the total metal ion and H2L volume remained constant (4 ml). CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as solvent at pH 7.2 using HEPES buffer. Job's plots were drawn by plotting ΔF versus mole fraction of Zn2+ and Cu2+.
1, v/v) was used as solvent at pH 7.2 using HEPES buffer. Job's plots were drawn by plotting ΔF versus mole fraction of Zn2+ and Cu2+.
Computational method
All calculations were carried out at the B3LYP34 level using Gaussian 09 software package.35 The 6-31G(d,p) basis set was assigned for the elements except for zinc and copper. The LANL2DZ basis set, with an effective core potential for Zn and Cu, was used.36 Vertical electronic excitations based on B3LYP optimized geometries were computed using the time-dependent density functional theory (TDDFT) formalism37 in acetonitrile using conductor-like polarizable continuum model (CPCM).38Conclusions
Thus we have successfully developed a new coumarin based chemosensor for the selective dual sensing of Cu2+ and Zn2+ over other metal ions. The receptor H2L shows about 6 fold increase in fluorescent intensity upon addition of Zn2+ and also exhibits quenching of emission intensity upon addition of Cu2+ without the interference of other metal ions present in solution. It exhibits two sets of integrated logic gates: (a) one INHIBIT logic gate with Zn2+ and Cu2+ or Zn2+ and EDTA as chemical inputs (b) one IMPLICATION logic gate with Cu2+ and EDTA as chemical inputs. We belief that in near future, our designed chemosensor H2L will lead to several important openings in the synthesis of other important chemosensors with additional application in biological systems.Acknowledgements
Financial supports received from the Department of Science and Technology, New Delhi, India (no. SB/EMEQ–242/2013) is gratefully acknowledged. D. Sarkar and A. K. Pramanik are thankful to CSIR, New Delhi, India, for their fellowships.Notes and references
- (a) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS PubMed; (b) M. Li, H. Ge, R. L. Arrowsmith, V. Mirabello, S. W. Botchway, W. Zhu, S. I. Pascu and T. D. James, Chem. Commun., 2014, 50, 11806 RSC.
- (a) Z. Dai and J. W. Canary, New J. Chem., 2007, 31, 1708 RSC; (b) K. Jobe, C. H. Brennan, M. Motevalli, S. M. Goldup and M. Watkinson, Chem. Commun., 2011, 47, 6036 RSC.
- H. Scherz and E. Kirchhoff, J. Food Compos. Anal., 2006, 19, 420 CrossRef CAS PubMed.
- P. C. Bull, G. R. Thomas, J. M. Rommens, J. R. Forbes and D. W. Cox, Nat. Genet., 1993, 5, 327 CrossRef CAS PubMed.
- (a) P. Li, X. Duan, Z. Chen, Y. Liu, T. Xie, L. Fang, X. Li, M. Yin and B. Tang, Chem. Commun., 2011, 47, 7755 RSC; (b) N. J. Robinson and D. R. Winge, Biochemistry, 2010, 79, 537 CrossRef CAS PubMed.
- T. Finkel, M. Serrano and M. A. Blasco, Nature, 2007, 448, 767 CrossRef CAS PubMed.
- Q. Zou, X. Li, J. Zhang, J. Zhou, B. Sun and H. Tian, Chem. Commun., 2012, 48, 2095 RSC.
- Z. Q. Guo, W.-Q. Chen and X.-M. Duan, Org. Lett., 2010, 12, 2202 CrossRef CAS PubMed.
- S. Dalapati, S. Jana, M. A. Alam and N. Guchhait, Sens. Actuators, B, 2011, 160, 1106 CrossRef CAS PubMed.
- C. Vulpe, B. Levinson, S. Whitney, S. Packman and J. Gitschier, Nat. Genet., 1993, 3, 7 CrossRef CAS PubMed.
- S. H. Hahn, M. S. Tanner, D. M. Danke and W. A. Gahl, Biochem. Mol. Med., 1995, 54, 142 CrossRef CAS.
- (a) K. Shen, X. Yang, Y. Cheng and C. Zhu, Tetrahedron, 2012, 68, 5719 CrossRef CAS PubMed; (b) L. Zhao, L. Yang, C. He, J. Wang and C. Duan, Dalton Trans., 2014, 43, 335 RSC; (c) T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 141 RSC; (d) Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568 RSC; (e) D. Buccella, J. A. Horowitz and S. J. Lippard, J. Am. Chem. Soc., 2011, 133, 4101 CrossRef CAS PubMed.
- (a) D. Sarkar, A. K. Pramanik and T. K. Mondal, RSC Adv., 2014, 4, 25341 RSC; (b) D. Sarkar, A. K. Pramanik and T. K. Mondal, J. Lumin., 2014, 146, 480 CrossRef CAS PubMed; (c) S. S. Mati, S. Chall, S. Konar, S. Rakshit and S. C. Bhattacharya, Sens. Actuators, B, 2014, 201, 204 CrossRef CAS PubMed; (d) S. Goswami, A. K. Das, K. Aich, A. Manna, S. Maity, K. Khanra and N. Bhattacharyya, Analyst, 2013, 138, 4593 RSC.
- (a) P. Kaur, S. Kaur, K. Singh, P. R. Sharma and T. Kaur, Dalton Trans., 2011, 40, 10818 RSC; (b) J. Huang, M. Liu, X. Ma, Q. Dong, B. Ye, W. Wang and W. Zeng, RSC Adv., 2014, 4, 22964 RSC.
- (a) R. Azadbakht, H. Keypour, H. A. Rudbari, A. H. Mohammad Zaheri and S. Menati, J. Lumin., 2012, 132, 1860 CrossRef CAS PubMed; (b) H. Song, S. Rajendiran, E. Koo, B. K. Min, S. K. Jeong, T. D. Thangadurai and S. Yoon, J. Lumin., 2012, 132, 3089 CrossRef CAS PubMed; (c) S. Kotha, D. Goyal, S. Banerjee and A. Datta, Analyst, 2012, 137, 2871 RSC.
- (a) R. Alam, T. Mistri, A. Katarkar, K. Chaudhuri, S. K. Mandal, A. R. Khuda-Bukhsh, K. K. Das and M. Ali, Analyst, 2014, 139, 4022 RSC; (b) M. Shellaiah, Y.-H. Wu and H.-C. Lin, Analyst, 2013, 138, 2931 RSC; (c) H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 3102 RSC; (d) A. Liu, L. Yang, Z. Zhang, Z. Zhang and D. Xu, Dyes Pigm., 2013, 99, 472 CrossRef CAS PubMed; (e) J. Q. Ang, B. T. T. Nguyen and C.-S. Toh, Sens. Actuators, B, 2011, 157, 417 CrossRef CAS PubMed; (f) S. Goswami, S. Das, K. Aich, D. Sarkar and T. K. Mondal, Tetrahedron Lett., 2013, 54, 6892 CrossRef CAS PubMed; (g) S. Goswami, S. Paul and A. Manna, RSC Adv., 2013, 3, 25079 RSC.
- K. Szacilowski, Chem. Rev., 2008, 108, 3481 CrossRef CAS PubMed.
- (a) X. Chen, Z. Li, Y. Xiang and A. Tong, Tetrahedron Lett., 2008, 49, 4697 CrossRef CAS PubMed; (b) U. Pischel and B. Heller, New J. Chem., 2008, 32, 395 RSC; (c) T. Gupta and M. E. van der Boom, Angew. Chem., Int. Ed., 2008, 47, 5322 CrossRef CAS PubMed.
- (a) A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574 CrossRef PubMed; (b) U. Pischel, Angew. Chem., Int. Ed., 2007, 46, 4026 CrossRef CAS PubMed.
- K. Rurack, C. Trieflinger, A. Koval'chuck and J. Daub, Chem.–Eur. J., 2007, 13, 8998 CrossRef CAS PubMed.
- (a) L. Zhao, D. Sui, J. Chai, Y. Wang and S. Jiang, J. Phys. Chem. B, 2006, 110, 24299 CrossRef CAS PubMed; (b) L. Zhao, Q. Hou, D. Sui, Y. Wang and S. Jiang, Spectrochim. Acta, Part A, 2007, 67, 1120 CrossRef PubMed; (c) L. Zhao, S. Wang, Y. Wu, Q. Hou, Y. Wang and S. Jiang, J. Phys. Chem. C, 2007, 111, 18387 CrossRef CAS.
- D. Sarkar, A. Pramanik, S. Biswas, P. Karmakar and T. K. Mondal, RSC Adv., 2014, 4, 30666 RSC.
- (a) D. Ray and P. K. Bharadwaj, Inorg. Chem., 2008, 47, 2252 CrossRef CAS PubMed; (b) N. C. Lim, J. V. Schuster, M. C. Porto, M. A. Tanudra, L. L. Yao, H. C. Freake and C. Bruckner, Inorg. Chem., 2005, 44, 2018 CrossRef CAS PubMed; (c) H. E. Katerinopoulos, Curr. Pharm. Des., 2004, 10, 3835 CrossRef CAS.
- (a) M. Suresh and A. Das, Tetrahedron Lett., 2009, 50, 5808 CrossRef CAS PubMed; (b) L. Cao, C. Jia, Y. Huang, Q. Zhang, N. Wang, Y. Xue and D. Du, Tetrahedron Lett., 2014, 55, 4062 CrossRef CAS PubMed; (c) H. A. El-Shekheby, A. H. Mangood, S. M. Hamza, A. S. Al-Kady and E. M. Ebeid, Luminescence, 2014, 29, 158 CrossRef CAS PubMed; (d) J. Hou, B. Liu, K. Li, K. Yu, M. Wu and X. Yu, Talanta, 2013, 116, 434 CrossRef CAS PubMed; (e) H. Wang, Y. Feng and S. Meng, J. Chem. Res., 2012, 36, 587 CrossRef CAS; (f) J. Wu, R. Sheng, W. Liu, P. Wang, H. Zhang and J. Ma, Tetrahedron, 2012, 68, 5458 CrossRef CAS PubMed; (g) N. Chattopadhyay, A. Mallick and S. Sengupta, J. Photochem. Photobiol., A, 2006, 177, 55 CrossRef CAS PubMed.
- S. Wang, G. Men, L. Zhao, Q. Hou and S. Jiang, Sens. Actuators, B, 2010, 145, 826 CrossRef CAS PubMed.
- Y. Liu, Q. Fei, H. Shan, M. Cui, Q. Liu, G. Feng and Y. Huan, Analyst, 2014, 139, 1868 RSC.
- L. Qua, C. Yina, F. Huob, J. Chaob, Y. Zhang and F. Cheng, Sens. Actuators, B, 2014, 191, 158 CrossRef PubMed.
- M. V. López, M. E. Vázquez, C. Gómez-Reino, R. Pedrido and M. R. Bermejo, New J. Chem., 2008, 32, 1473 RSC.
- (a) A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2002, 8, 4935 CrossRef; (b) Y. Fu, Q. Feng, X. Jiang, H. Xu, M. Li and S. Zang, Dalton Trans., 2014, 43, 5815 RSC; (c) D. C. Magri, M. C. Fava and C. J. Mallia, Chem. Commun., 2014, 50, 1009 RSC; (d) S. Wang, G. Men, L. Zhao, Q. Hou and S. Jiang, Sens. Actuators, B, 2010, 145, 826 CrossRef CAS PubMed.
- (a) A. Brahmia, T. B. Ayed and R. B. Hassen, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, 69, o1296 CAS; (b) T. Shibahara, M. Takahashi, A. Maekawa and H. Takagi, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, o429 CAS.
- (a) A. J. Moghadam, R. Omidyan, V. Mirkhani and G. Azimi, J. Phys. Chem. A, 2013, 117, 718 CrossRef PubMed; (b) Z. Wang, D. M. Friedrich, C. C. Ainsworth, S. L. Hemmer, A. G. Joly and M. R. Beversluis, J. Phys. Chem. A, 2001, 105, 942 CrossRef CAS; (c) I. Presiado, Y. Erez, R. Gepshtein and D. Huppert, J. Phys. Chem. C, 2010, 114, 3634 CrossRef CAS; (d) F. Wua, L. Ma, S. Zhanga, Y. Genga, J. Lüa and X. Chenga, Chem. Phys. Lett., 2012, 141, 519 Search PubMed.
- (a) K. M. K. Swamy, S. K. Ko, S. K. Kwon, H. N. Lee, C. Mao, J. M. Kim, K. H. Lee, J. Kim, I. Shin and J. Yoon, Chem. Commun., 2008, 5915 RSC; (b) H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS PubMed.
- N. Hamdi, C. Fischmeister, M. C. Puerta and P. Valerga, Med. Chem. Res., 2011, 20, 522 CrossRef CAS.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS; (c) D. Andrae, U. Haeussermann, M. Dolg, H. Stoll and H. Preuss, Theor. Chim. Acta, 1990, 77, 123 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision D.01, Gaussian Inc., Wallingford CT, 2009 Search PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef CAS PubMed.
- (a) R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett., 1996, 256, 454 CrossRef CAS; (b) R. E. Stratmann, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 1998, 109, 8218 CrossRef CAS PubMed; (c) M. E. Casida, C. Jamorski, K. C. Casida and D. R. Salahub, J. Chem. Phys., 1998, 108, 4439 CrossRef CAS PubMed.
- (a) V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995 CrossRef CAS; (b) M. Cossi and V. Barone, J. Chem. Phys., 2001, 115, 4708 CrossRef CAS PubMed; (c) M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Association constant determination, detection limit determination, 1H NMR, HRMS, UV-vis titration spectra of HL with different metal ions etc. See DOI: 10.1039/c4ra12920b |
| This journal is © The Royal Society of Chemistry 2015 |