Preparation of layered graphitic carbon nitride/montmorillonite nanohybrids for improving thermal stability of sodium alginate nanocomposites†
Abstract
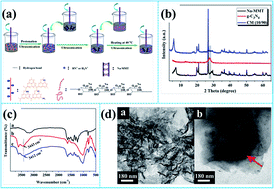
Graphitic carbon nitride (g-C3N4)/montmorillonite (Na-MMT) nanohybrids were successfully fabricated by a facile mixing strategy and then introduced into a sodium alginate host to prepare nanocomposites with different loading levels using a casting technique. The introduction of 4.0 wt% nanohybrids led to the maximal improvement by ca. 149 °C in thermal stability. The improvement of the thermal stability was due to the reason that the mutual intercalation weakened the interlayered interaction of g-C3N4 nanosheets or Na-MMT sheets and enhanced the physical barrier effect that retarded the permeation of heat and the escape of volatile products. This work may provide an approach for exploiting g-C3N4 based materials.

 Please wait while we load your content...
Please wait while we load your content...