Tweaking anisotropic gold nanostars: covariant control of a polymer–solvent mixture complex†
Abhitosh Kediaa,
Harsh Kumarb and
Pandian Senthil Kumar*a
aDepartment of Physics & Astrophysics, University of Delhi, Delhi 110007, India. E-mail: pskumar@physics.du.ac.in
bUSIC, University of Delhi, Delhi 110007, India
First published on 2nd December 2014
Abstract
A binary solvent mixture (N,N-dimethylformamide (DMF)–propanol in the present case) has been employed for the first time in a simple yet versatile approach for the in situ intuitive and highly tweaked formation of complex anisotropic gold nanostructures. In this novel approach, the uncommon usage of a polar/non-polar binary solvent mixture provides extra degrees of freedom in tailoring the intrinsic solution properties via simultaneously modifying dipole–dipole and H-bonding interactions with the universally soluble amphiphilic polymer PVP. Interactions are carefully monitored through NMR, which further establishes a rational paradigm in better understanding the kinetic control over both nucleation and size/shape transition/evolution of reproducible anisotropic Au nanostructures, as revealed/correlated through optical absorption and TEM measurements respectively. This unusual size/shape transformation strategy for gold nanostructures allows precise tuning of their respective plasmonic characteristics over the entire visible/NIR spectral range, significantly enabling them to serve as excellent candidates/substrates for tunable surface enhanced Raman spectroscopy (SERS), the preliminary measurements of which are systematically illustrated.
Introduction
Owing to the highly specific size/shape dependent physico-chemical properties of metal nanoparticles (NPs), such as their localized surface plasmon resonance (SPR) or high catalytic activity,1–7 relentless extra care needs to be taken in stabilizing/tweaking the aesthetics of the asymmetric metal NPs with complex morphologies (such as polyhedra, multipods, star/flower etc.).8–19 Vast numbers of literature reports are available on the tweaking/shaping of controlled gold nanostructures (with/without seed mediation) utilizing routinely a variety of surfactants/polymers like CTAB/PVP/2-[4-(2-hydroxyethyl)-1-piperazinylethane-sulfonic acid (HEPES) etc., as shape directing/stabilizing agents.8–11,15–17,20 In some selective reports, alternative approaches are demonstrated such as: the gradual size tuning of citrate-reduced Au nanospheres via a change in Cl− ion concentration in solution;21 synthesis of multiple shaped Au by different dendritic peptides;22 precise control of size and nanostructuration of multi-branched Au NPs by changing the HEPES concentration;20,23 and very recently, tuning the plasmonic response of Au nanostar arrays from visible to near infrared by electron beam lithography.24 Understanding the morphological transition mechanism in noble metal NPs becomes an inevitable tool for not only engineering their plasmonic response from the visible to the far IR region of the EM spectrum,19–28 but also for extending their application potential from plasmonics to nanophotonics to biosensing and SERS etc.1–5So far, most of the successful colloidal synthetic procedures for the production of monodisperse/uniform colloidal gold nanoparticles have utilized an aqueous medium but synthetic schemes in organic media have also been reported recently.10–18 Among these, DMF, a polar aprotic organic solvent well-known for its high synthetic value, and good chemical and thermal stability (even at its boiling point, 153.8 °C), has been widely chosen for the preparation of anisotropic gold nanostructures with a variety of geometrical shapes.16,18,29,30 In conjunction with PVP, the high polar DMF solvent has been successfully utilized in tweaking/morphological characteristics such as the size/shape of complex 3D gold nanostars in a controlled manner using the well known seed mediated synthesis by varying the size as well as concentration of the seed particle in the growth solution,31,32 which induces considerable changes in the core size, the number, length and broadening of the branches. The plasmonic response of such gold nanostructures is extended well towards the near IR region due to the presence of sharp edges/tips, where strong localization of electromagnetic field exists, suggesting strong application potentials in the fields of nanoantennas and nanophotonics.33,34 The nanoscale control of surface roughness/branching, ranging from planar anisotropic to complex elongated spikes, allows precise tuning of the nanoparticle optical properties with a high level of accuracy. Such anisotropic nanostructures were demonstrated to be excellent candidates for SERS applications.35–39
In our previous reports, we have already discussed in detail that increasing the pH of the reaction mixture conveniently results in fine tuning of the plasmon band as well as the morphology of gold nanostars in a single step synthetic protocol.28 Furthermore, the solvent interaction with PVP has been reiterated as a crucial factor.17 We illustrate that the solvent–solute interaction dominates the nucleation regime and additionally plays a significant role in the polymer chemisorption onto the surface of the metal nanoparticles so directing/shaping them to grow anisotropically with different intriguing sizes and shapes.
Convinced by the fact that the conformational changes of PVP in different solvents lead to different sized and shaped Au nanostructures17 and in order to simplify the two-step seed mediated synthesis procedures for tuning the metal nanostructure morphology,31,32,40 herein, we utilized a novel versatile single step synthesis protocol, involving the use of a binary solvent mixture, such as DMF–2-propanol. To the best of our knowledge, this is the first time such a method has been used in the sequential tuning of gold nanostars, in sharp contrast with the formation of regular structures such as the nanocubes41 and nanowires42 as reported earlier. The good donor/acceptor properties of DMF enable it to dissolve a wide range of polar/non-polar solvents and its physico-chemical properties have been studied in detail in conjunction with various solvents like water, alcohol etc., where the nature of molecular interactions between DMF and other solvents were carefully discussed in detail.43–47 The introduction of 2-propanol into DMF changes its H-bonding tendency as well as dipole–dipole interactions, which play a crucial role in governing the conformational changes of PVP, thus determining its reducing ability. This provides the necessary extra degrees of freedom for the controlled tuning of the plasmonic response of gold nanostars in a sequential manner from the visible to the near IR region and can be used for specific applications such as surface enhancement Raman scattering (SERS).
Materials and methods
In a typical synthesis, 24 μl aqueous solution of hydrochloroauric acid (HAuCl4·3H2O, Aldrich) at a concentration of 0.17 M was mixed homogenously with 10 mM polyvinylpyrrolidone (PVP average MW = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aldrich) solution in 15 ml dimethylformamide (DMF, Merck) and continuously stirred in order to obtain gold nanostars in a seedless colloidal synthesis procedure. Further details of the synthesis procedure and the mechanism of formation of gold nanostars is discussed in our previous work.18 In the present work, the influence of 2-propanol was studied by adding it into DMF in different molar ratios before the addition of PVP and the metal precursor. The molar ratio of PVP to metal ions (calculated in terms of polymer repeating unit or monomer chain length) was kept at ∼3250 in all our experimental conditions. A change in the color of solutions indicated the formation of gold nanostructures. All experiments and the various sample preparation procedures were carried out at room temperature, unless otherwise mentioned. Henceforth, 2-propanol will be referred to as propanol.
000, Aldrich) solution in 15 ml dimethylformamide (DMF, Merck) and continuously stirred in order to obtain gold nanostars in a seedless colloidal synthesis procedure. Further details of the synthesis procedure and the mechanism of formation of gold nanostars is discussed in our previous work.18 In the present work, the influence of 2-propanol was studied by adding it into DMF in different molar ratios before the addition of PVP and the metal precursor. The molar ratio of PVP to metal ions (calculated in terms of polymer repeating unit or monomer chain length) was kept at ∼3250 in all our experimental conditions. A change in the color of solutions indicated the formation of gold nanostructures. All experiments and the various sample preparation procedures were carried out at room temperature, unless otherwise mentioned. Henceforth, 2-propanol will be referred to as propanol.
Optical absorption measurements were recorded in all our as-prepared nanoparticle solution samples in the wavelength range of 200–1100 nm using a Thermo Scientific absorption spectrophotometer. The 13C NMR spectra were recorded on a Bruker Avance-400 spectrometer using TMS as the internal standard and CDCl3 as a NMR solvent (chemical shifts in ppm). TEM samples were prepared by drying the five-fold centrifuged samples in ethanol at around 4500 rpm (to remove excess PVP) on carbon formvar coated copper grids and the images were acquired using a FEI-Technai G2 system operated at an accelerating voltage of 300 kV. The surface enhanced Raman scattering (SERS) spectra were obtained using a Renishaw inVia Raman spectrometer with 785 nm He–Ne laser source. Samples for SERS were prepared by dispersing five-fold centrifuged Au nanoparticles in chloroform and spreading them to form a monolayer at the air–water interface. They were lifted onto the silicon substrates before the drop-cast deposition of 10−6 M of the dye molecule, crystal violet. The SERS spectra were obtained for two different positions of each of the samples and averages were plotted.
Results and discussion
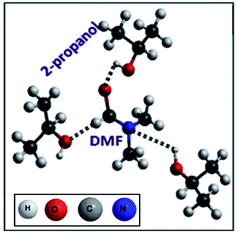
Fig. 1 shows the 13C NMR spectra of DMF and propanol at different mole fractions (MF) of DMF with respect to propanol concentration. At high MF of DMF i.e., at low concentration of propanol in DMF, the 13C carbonyl chemical shift of DMF increases slightly compared with pure DMF (see Fig. 1 for peak values) signifying the changes occurring in the self-association equilibria of DMF, which are evidently due to the change in dipole–dipole interactions along with the formation of hydrogen bonds, O–H (of propanol)⋯N(CH3)2 or O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN of DMF along with H-bonding between H–O of propanol and the formyl proton of DMF as schematically shown in Scheme 1. With a further decrease in the MF of DMF, the 13C carbonyl chemical shift of DMF increases more rapidly (see Fig. 1 for peak values), demonstrating the fact that DMF–propanol hydrogen bonding increases with increasing alcohol content, and DMF is surrounded by more and more propanol molecules, similar to the case of DMF in methanol;45 propanol can be visualized as a watermelon in which the DMF molecules act as seeds. The observed results indicate the existence of intermolecular associations between O–H groups of alcohol and C
CN of DMF along with H-bonding between H–O of propanol and the formyl proton of DMF as schematically shown in Scheme 1. With a further decrease in the MF of DMF, the 13C carbonyl chemical shift of DMF increases more rapidly (see Fig. 1 for peak values), demonstrating the fact that DMF–propanol hydrogen bonding increases with increasing alcohol content, and DMF is surrounded by more and more propanol molecules, similar to the case of DMF in methanol;45 propanol can be visualized as a watermelon in which the DMF molecules act as seeds. The observed results indicate the existence of intermolecular associations between O–H groups of alcohol and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of DMF along with DMF–DMF and alcohol–alcohol interactions as reported earlier.47
O groups of DMF along with DMF–DMF and alcohol–alcohol interactions as reported earlier.47
 | ||
| Fig. 1 13C NMR spectra of DMF and DMF–propanol mixtures in a selective range at different MF of DMF as shown. | ||
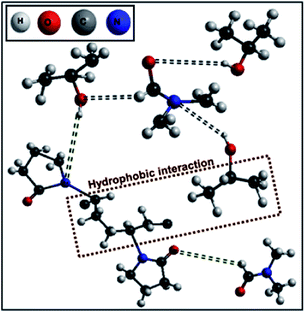
Moreover, the interactions between PVP–DMF and PVP–propanol systems were individually discussed in detail in our previous work,17 which talks about the dominant dipole–dipole interaction in the PVP–DMF system as well as the hydrophobic interaction between PVP and propanol. In the present case, PVP addition to the DMF–propanol composition (for different MF) results in the slow but steady re-organization of the polymer network, as systematically depicted in Scheme 2 in accordance with the 13C NMR studies (Fig. 2). A slight but definite downfield shift of the carbonyl peak (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak around 175 ppm)48 of PVP with the decrease in MF of DMF (see Fig. 2 for peak values) signifies the weakening of H-bonding between DMF and propanol along with the initiation of hydrophobic interactions between propanol and long chain PVP molecules through hydrogen bond formation between the nitrogen of PVP and the HO–C of propanol as portrayed in Scheme 2. The slight up-field chemical shift in the DMF carbonyl peak and the corresponding downfield shift in the C–OH bond of propanol (with increase in propanol volume) in the DMF–propanol–PVP system in comparison to DMF–propanol exemplifies the loosening of DMF–PVP bonding in conjunction with increasing PVP–propanol H-bonding interactions which ostensibly augment the incremental increase in the hydrophobic interactions assimilated through the long polymer chain structure and the relatively smaller alcohol group. The effects are at their maximum for the pure PVP–propanol system.
O peak around 175 ppm)48 of PVP with the decrease in MF of DMF (see Fig. 2 for peak values) signifies the weakening of H-bonding between DMF and propanol along with the initiation of hydrophobic interactions between propanol and long chain PVP molecules through hydrogen bond formation between the nitrogen of PVP and the HO–C of propanol as portrayed in Scheme 2. The slight up-field chemical shift in the DMF carbonyl peak and the corresponding downfield shift in the C–OH bond of propanol (with increase in propanol volume) in the DMF–propanol–PVP system in comparison to DMF–propanol exemplifies the loosening of DMF–PVP bonding in conjunction with increasing PVP–propanol H-bonding interactions which ostensibly augment the incremental increase in the hydrophobic interactions assimilated through the long polymer chain structure and the relatively smaller alcohol group. The effects are at their maximum for the pure PVP–propanol system.
 | ||
| Fig. 2 13C NMR spectra of PVP and PVP–DMF–propanol mixtures in a selective range at different MF of DMF. | ||
In contrast, when HAuCl4 is added to this PVP–binary solvent mixture, instantaneous solvation as well as complexation of the dynamically unstable PVP–AuCl4− effectively takes place owing to the presence of the lone pairs of electrons in the oxygen and nitrogen atoms of the entangled PVP network, sequentially disproportionating themselves to form Au0 species in the absence of any other external reducing agent or energy resources. These in situ formed tiny gold seed particles then compete with the polymer, PVP (the affinity of which decreases as the MF of DMF decreases in the whole composition range used in the present case), thereby desolvating the surrounding DMF molecules in addition to weakening the PVP–propanol hydrogen bonding (as shown from the upfield chemical shifts of PVP, DMF and propanol carbonyl peaks in Fig. 3 in the presence of the Au nanoparticles in comparison to the PVP–DMF–propanol mixture shown in Fig. 2). In contrast, upon addition of the gold precursor to the PVP–propanol system, the observed slight downfield chemical shift of the PVP carbonyl peak (with respect to PVP–propanol, see Fig. 2 and 3) has been attributed to the fact that the hydrogen bonding between PVP and propanol remains almost intact and is further dominated by strong hydrophobic interactions. This kind of composite reduction along with steric stabilization of PVP by the gold seed particles in the binary solvent system exclusively result in the steady/stable anisotropic nanoparticle growth, which has not been possible with any other conventional colloidal growth techniques.
 | ||
| Fig. 3 13C NMR spectra of PVP and Au nanoparticles in a selective range obtained at different MF of DMF. | ||
Thus, the relevant stabilization of the as-prepared gold nanoparticle morphology was investigated utilizing transmission electron microscopy (TEM) in the light of distinct molecular interactions existing between DMF–propanol–PVP mixtures as discussed above.
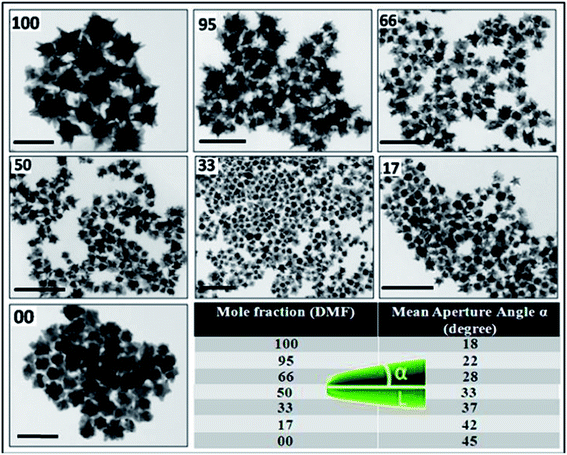
In comparison with the gold nanostars synthesized in pure DMF, where the tips/spikes are longer and sharper, the sequential addition of propanol to DMF (i.e. decrease in MF of DMF) brings sequential changes in the sharpness, length and size of the tips/spikes as demonstrated clearly in Fig. 4. The proposed aperture angle α increases sequentially, signifying the size/shape poly-dispersion as well as the explicit broadening of tips/spike-like features at the expense of their number density along with a decrease in the overall size of the as-formed gold nanostructures, as clearly seen from the high magnification TEM images in Fig. 4. Further measurements under different TEM tilt angles would be of great interest in eliminating the so-called projection effect and for necessary quantification of the obtained data.
Such visible structural changes in the morphology of the gold nanoparticles can be easily justified in terms of the chronological changes in the optical response as seen through UV-Vis spectroscopy. Thus, simple addition of an external alcohol like propanol into DMF sequentially tweaks the longitudinal localized surface plasmon resonance (LSPR) peaks from 930 nm (1.33 eV, for pristine Au nanostars) to 650 nm (1.87 eV) in a precisely reproducible manner, as shown in Fig. 5a. Although the gold nanoparticle morphology tweaking has been successfully carried out by various research groups worldwide through a complicated multi-step synthetic process,31,32,40 our present colloidal solution synthesis stands apart for its inherent simplicity and ease of reproducibility as well as for the quality and quantity of nanoparticles produced.
At higher propanol concentrations, gold seed particles are nucleated at a faster rate resulting in the blue shifting of the LSPR with a decrease in overall size of the as-formed gold nanostructures.
It is well known that the plasmon resonance of gold nanostars results from the hybridization of core and tip mode plasmons or from the near base region of the tip.33,49 Based upon this fact, we analyzed the extinction spectra of the different gold nanostructures obtained by using various DMF–propanol ratios, utilizing the standard Gaussian multi-peak fitting procedure as illustrated in Fig. 5b with dashed lines (fitting the optical spectra results in a slight increase in intensity of the longitudinal plasmon peak). The main assessment inferred from here is that the intensity of the transverse LSPR mode (corresponding to the central core) steadily grows at the expense of the blue shifted longitudinal LSPR mode (related to the tips), in direct correlation with the observed HRTEM images (shown in Fig. 5c), strongly corroborating the fact that with increasing propanol concentration, the tip/spike-like gold nanostructures cease to grow in length, but rather expand in width, customarily reducing their overall size/number. Besides, the anti-bonding plasmon mode (occurring at 800 nm in the deconvoluted optical spectra of as prepared gold nanostars) starts blue shifting with a reduction in peak intensity (see Fig. 5c, where the defect free single nanoparticle morphology is shown), until the MF of DMF reaches 50, emphasizing the weakened coupling strength between the core and the tip mode plasmons, which correlates well with the broadening as well as the decrease in the overall number of tips in a single nanoparticle. As the MF of DMF decreases below 50, the significant anti-bonding plasmon mode appears again, depicted visually in the change in the shape of the absorption spectra itself, probably due to the inheritance of the crystal lattice defects in the nanoparticle morphology with reference to the dynamical changes in the applied synthetic procedure (see Fig. 5a and b).
Thus, the present results are in complete contrast with our earlier work involving the addition of NaOH into the DMF–PVP mixture,28 which had a high impact on the reaction kinetics due to the significant oxidation of inserted –OH species in between or at the end of the PVP polymer chain, thereby fastening the nucleation of seed particles, and eventually ripening them in the form of planar elongated or spheroidal gold nanostructures. The mixing of DMF with an alcohol not only induces changes in the dipolar interactions within DMF, but also disrupts either partially or completely the self association of propanol, thereby allowing the essential formation of H-bonds between alcohol and the DMF. These miscible weak interactions further articulate the hydrophobic/hydrophilic end chain imbalance in the PVP conformation, thus leading to the smoothening of the nucleation of seed particles, which invariably results in the congruent size/shape tuning of the gold nanostars, as collectively summarized in Scheme 3: DMF interacts with propanol by virtue of its better hydrogen bond acceptor ability resulting in both structural as well as packing effects.
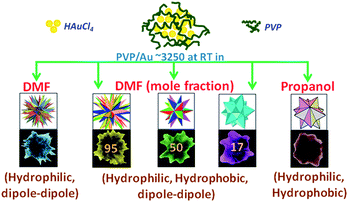 | ||
| Scheme 3 Sequential size/shape tweaking of the gold nanostars with reference to systematic changes in the molecular interactions of PVP with the reaction mixture. | ||
Yet, both the above mentioned synthetic protocols result in tweaking the plasmonic response (blue shifting of LSPR) which is in good correlation with the morphology of the gold nanostars although the driving forces for the changes are poles apart. In the present case of the binary solvent mixture, the reaction is not dominated by kinetic parameters, easing the plausible formation of planar elongated/spheroidal gold nanostructures, while it is strongly favorable using NaOH at higher concentrations (as this reaction is governed by kinetic parameters).
In tune with the literature reports supporting the fact that metal colloidal nanoparticles were able to enhance the SERS to a very large extent,35–39,50–52 we have carefully analyzed the SERS data (Fig. 6) using the analyte, crystal violet (CV, at a concentration of 10−6 M) coated on the monolayer surface of our as-prepared gold nanostructures. The various enhanced Raman peaks of the dye molecule CV37,53 are tabulated with reference to their molecular origin as shown in Appendix Table A1.†
 | ||
| Fig. 6 SERS Raman spectra of 10−6 M crystal violet without and with Au nanostructures obtained at different MF of DMF). The TEM image scale bar is 20 nm. | ||
The corresponding Raman signals of CV were greatly enhanced; lower concentrations (say, 10−6 M) of CV could not be detected without the assistance of a proper plasmonic substrate. Enhancement factors (EF) are calculated for different samples obtained at different mole fractions of DMF (see Table 1). A detailed discussion on determining the SERS EFs is given in the ESI† section based upon the work of Stefania Mura et al.54 Assuming that all the CV molecules contribute equally, it has been demonstrated that the maximum SERS enhanced signal is obtained for pristine gold nanostars (Table 1), which first decreases (until the MF of DMF = 33) and eventually starts increasing with higher propanol concentration (Table 1).
| DMF (MF) | Enhancement factor (EE) |
|---|---|
| 100 | 1.90 × 104 |
| 95 | 0.60 × 104 |
| 66 | 0.34 × 104 |
| 50 | 0.43 × 104 |
| 33 | 0.31 × 104 |
| 17 | 0.50 × 104 |
| 00 | 1.20 × 104 |
The strong enhancements observed for the central carbon atom, nitrogen atoms and π electrons in the phenyl ring of the dye molecule, crystal violet, suggest strong substrate–analyte interactions, which intrinsically depend upon two major factors: the surface roughness of the nanoparticles and the inter-plasmon coupling-junction between two nanoparticles. Hence, in the case of pristine gold nanostars, the EM field is maximum because of the inherent surface roughness of the nanoparticles, whereas in the case of nanostars in pure propanol, the smaller particle size/higher surface area results in the formation of an increased number of possible “hot-spots” (see Appendix Fig. A1†). Consequently, we firmly believe that the precisely controlled nanoparticle morphology along with their structurally modified rearrangement on a substrate is largely responsible for maximizing the SERS signals. Further work is in progress in our research group to quantitatively identify the specific role of the plasmonic substrate as well as the analyte in the SERS enhancement in terms of excitation wavelength, different analytes/concentration etc.
Conclusions
We have utilized a versatile room temperature colloidal synthesis protocol not only for controlling the size and shape of anisotropic gold nanostructures, but also for precisely engineering their plasmonic response over the entire visible/NIR spectral range. The protocol allows fine tuning of metal nanocrystals, which has not been possible with conventional nanocrystal growth methods or realized with such ease using existing patterning/lithographic techniques. The careful selection of the binary solvent mixture along with the modified molecular characteristics of PVP establishes a rational paradigm in better understanding the size/shape evolution of reproducible Au nanostructure morphology, as consistently revealed through optical absorption and TEM. The preliminary SERS measurements on the plasmonic substrates prepared using the air–water interfacial monolayer of the as-prepared PVP-coated Au nanostructures were systematically illustrated using the representative analyte, crystal violet.Acknowledgements
One of the authors AK thanks CSIR for the NET-SRF fellowship. We sincerely thank the R&D Scheme, MTech (Nano) and USIC at the University of Delhi, for materials characterization. We are grateful to Mr Rahul Bharadwaj for TEM imaging and Mr Amit for NMR measurements.References
- K. A. Willets and R. P. V. Duyne, Localized Surface Plasmon Resonance Spectroscopy and Sensing, Annu. Rev. Phys. Chem., 2007, 58, 267 CrossRef CAS PubMed
.
- J. A. Schuller, E. S. Barnard, W. Cai, Y. C. Jun, J. S. White and M. L. Brongersma, Plasmonics for Extreme Light Concentration and Manipulation, Nat. Mater., 2010, 9, 193 CrossRef CAS PubMed
.
- S. A. Maier and H. A. Atwater, Plasmonics: Localization and Guiding of Electromagnetic Energy in Metal/Dielectric Structures, J. Appl. Phys., 2005, 98, 011101 CrossRef PubMed
.
- M. L. Brongersma and P. G. Kik, Surface Plasmon Nanophotonics, Springer, 2007 Search PubMed
.
- V. Amendola, Synthesis of Gold and Silver Nanoparticles for Photonic Application, PhD thesis, University of Padova, Italy, 2008
.
- M. Rycenga, C. M. Cobley, W. L. Jie Zeng, C. H. Moran, D. Q. Qiang Zhang and Y. Xia, Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications, Chem. Rev., 2011, 111, 3669 CrossRef CAS PubMed
.
- D. Astruc, Nanoparticles and Catalysis, Wiley-VCHD, 2008 Search PubMed
.
- P. Herves, M. Perez-Lorenzo, L. M. Liz-Marzan, J. Dzubiella, Y. Lu and M. Ballauff, Catalysis by Metallic Nanoparticles in Aqueous Solution: Model Reactions, Chem. Soc. Rev., 2012, 41, 5577 RSC
.
- B. Lim and Y. Xia, Metal Nanocrystals with Highly Branched Morphologies, Angew. Chem., Int. Ed., 2011, 50, 76 CrossRef CAS PubMed
.
- A. R. Tao, S. Habas and P. Yang, Shape Control of Colloidal Metal Nanocrystals, Small, 2008, 4, 310 CrossRef CAS
.
- M. Grzelczak, J. Perez-Juste, P. Mulvaney and L. M. Liz-Marzan, Shape Control in Gold Nanoparticle Synthesis, Chem. Soc. Rev., 2008, 37, 1783 RSC
.
- T. K. Sau and A. L. Rogach, Nonspherical, Noble Metal Nanoparticles: Colloid-Chemical Synthesis and Morphology Control, Adv. Mater., 2010, 22, 1781 CrossRef CAS PubMed
.
- M. R. Langille, Chemical Methods for Controlling the Shape of Silver and Gold Nanocrystals, PhD thesis, Northwestern University, 2012
.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics?, Angew. Chem., Int. Ed., 2009, 48, 60 CrossRef CAS PubMed
.
- J. Zhang, H. Liu, Z. Wang and N. Ming, Shape-Selective Synthesis of Gold Nanoparticles with Controlled Sizes, Shapes, and Plasmon Resonances, Adv. Funct. Mater., 2007, 17, 3295 CrossRef CAS
.
- Y. Chen, X. Gu, C.-G. Nie, Z.-Y. Jiang, Z.-X. Xie and C.-J. Lin, Shape Controlled Growth of Gold Nanoparticles by a Solution Synthesis, Chem. Commun., 2005, 4181 RSC
.
- A. Kedia and S. K. Pandian, Solvent-Adaptable Poly(Vinylpyrrolidone) Binding Induced Anisotropic Shape Control of Gold Nanostructures, J. Phys. Chem. C, 2012, 116, 23721 CAS
.
- A. Kedia and S. K. Pandian, Precursor Driven Nucleation and Growth Kinetics of Gold Nanostars, J. Phys. Chem. C, 2012, 116, 1679 CAS
.
- H. Zhang, M. Jin and Y. Xia, Noble-Metal Nanocrystals with Concave Surfaces and Their Applications, Angew. Chem., Int. Ed., 2012, 51, 7656 CrossRef CAS PubMed
.
- G. Maiorano, L. Rizzello, M. A. Malvindi, S. S. Shankar, L. Martiradonna, A. Falqui, R. Cingolani and P. P. Pompa, Monodispersed and Size-Controlled Multibranched Gold Nanoparticles with Nanoscale Tuning of Surface Morphology, Nanoscale, 2011, 3, 2227 RSC
.
- L. Zhao, D. Jiang, Y. Cai, X. Ji, R. Xie and W. Yang, Tuning the Size of Gold Nanoparticles in the Citrate Reduction by Chloride Ions, Nanoscale, 2012, 4, 5071 RSC
.
- G. Palui, S. Ray and A. Banerjee, Synthesis of Multiple Shaped Gold Nanoparticles using
Wet Chemical Method by Different Dendritic Peptides at Room Temperature, J. Mater. Chem., 2009, 19, 3457 RSC
.
- J. A. Webb, W. R. Erwin, H. F. Zarick, J. Aufrecht, H. W. Manning, M. J. Lang, C. L. Pint and R. Bardhan, Geometry-Dependent Plasmonic Tunability and Photothermal Characteristics of Multibranched Gold Nanoantennas, J. Phys. Chem. C, 2014, 118, 3696 CAS
.
- M. Chirumamilla, A. Gopalakrishnan, A. Toma, R. P. Zaccaria and R. Krahne, Plasmon Resonance Tuning in Metal Nanostars for Surface Enhanced Raman Scattering, Nanotechnology, 2014, 25, 235303 CrossRef PubMed
.
- X. Xia, J. Zeng, B. Mcdearmon, Y. Zheng, Q. Li and Y. Xia, Silver Nanocrystals with Concave Surfaces and their Optical and Surface-Enhanced Raman Scattering Properties, Angew. Chem., Int. Ed., 2011, 50, 12542 CrossRef CAS PubMed
.
- E. C. Argibay, B. Rodriguez-Gonzalez, J. Pacifico, I. Pastoriza-Santos, J. Perez-Juste and L. M. Liz-Marzan, Chemical Sharpening of Gold Nanorods: The Rod-to-Octahedron Transition, Angew. Chem., Int. Ed., 2007, 119, 9141 CrossRef
.
- C. M. Aguirre, Reshaping the Metallic Surface of Gold Nanoshell, PhD thesis, Rice University, 2003
.
- A. Kedia and S. K. Pandian, Controlled Reshaping and Plasmon Tuning Mechanism of Gold Nanostars, J. Mater. Chem. C, 2013, 1, 4540 RSC
.
- I. Pastoriza-Santos and L. M. Liz-Marzan, N,N-Dimethylformamide as a Reaction Medium for Metal Nanoparticle Synthesis, Adv. Funct. Mater., 2009, 19, 679 CrossRef CAS
.
- A. V. Gaikwad, P. Verschuren, S. Kinge, G. Rothenberg and E. Eiser, Matter of Age: Growing Anisotropic Gold Nanocrystals in Organic Media, Phys. Chem. Chem. Phys., 2008, 10, 951 RSC
.
- S. Barbosa, A. Agrawal, L. Rodríguez-Lorenzo, I. Pastoriza-Santos, R. A. Alvarez-Puebla, A. Kornowski, H. Weller and L. M. Liz-Marzan, Tuning Size and Sensing Properties in Colloidal Gold Nanostars, Langmuir, 2010, 26, 14943 CrossRef CAS PubMed
.
- C. G. Khoury and T. Vo-Dinh, Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization, J. Phys. Chem. C, 2008, 112, 18849 CAS
.
- F. Hao, C. L. Nehl, J. H. Hafner and P. Nordlander, Plasmon Resonances of a Gold Nanostar, Nano Lett., 2007, 7, 729 CrossRef CAS PubMed
.
- R. Alvarez-Puebla, L. M. Liz-Marzan and F. J. G. D. Abajo, Light Concentration at the Nanometer Scale, J. Phys. Chem. Lett., 2010, 1, 2428 CrossRef CAS
.
- L. Rodríguez-Lorenzo, R. N. A. A. Lvarez-Puebla, F. J. G. D. Abajo and L. M. Liz-Marzan, Surface Enhanced Raman Scattering using Star-Shaped Gold Colloidal Nanoparticles, J. Phys. Chem. C, 2010, 114, 7336 Search PubMed
.
- J. Xie, Q. Zhang, J. Y. Lee and D. I. C. Wang, The Synthesis of SERS-Active Gold Nanoflower Tags for in Vivo Applications, ACS Nano, 2008, 2, 2473 CrossRef CAS PubMed
.
- E. N. Esenturk and A. R. H. Walker, Surface-Enhanced Raman Scattering Spectroscopy via Gold Nanostars, J. Raman Spectrosc., 2009, 40, 86 CrossRef CAS
.
- P. R. Sajanlal and T. Pradeep, Mesoflowers: A New Class of Highly Efficient Surface-Enhanced Raman Active and Infrared-Absorbing Materials, Nano Res., 2009, 2, 306 CrossRef CAS
.
- B. L. Sanchez-Gaytan, P. Swanglap, T. J. Lamkin, R. J. Hickey, Z. Fakhraai, S. Link and S. J. Park, Spiky Gold Nanoshells: Synthesis and Enhanced Scattering Properties, J. Phys. Chem. C, 2012, 116, 10318 CAS
.
- L. Rodríguez-Lorenzo, J. M. Romo-Herrera, J. Pérez-Juste, R. A. Alvarez-Puebla and L. M. Liz-Marzán, Reshaping and LSPR Tuning of Au Nanostars in the Presence of CTAB, J. Mater. Chem., 2011, 21, 11544 RSC
.
- S. Peng and Y. Sun, Synthesis of Silver Nanocubes in a Hydrophobic Binary Organic Solvent, Chem. Mater., 2010, 22, 6272 CrossRef CAS
.
- Q. Liu, Y. Zhou, W. Tu, S. Yan and Z. Zou, Solution-Chemical Route to Generalized Synthesis of Metal Germanate Nanowires with Room-Temperature, Light-Driven Hydrogenation Activity of CO2 into Renewable Hydrocarbon Fuels, Inorg. Chem., 2014, 53, 359 CrossRef CAS PubMed
.
- K. Ramachandran, P. Sivagurunathan, K. Dharmalingam and S. C. Mehrotra, Dielectric Relaxation Study of Amide–Alcohol Mixtures by Using Time Domain Reflectometry, Acta Phys.-Chim. Sin., 2007, 23, 1508 CrossRef CAS
.
- R. J. Sengwa, V. Khatri and S. Sankhla, Structure and H Bonding, in Binary Mixtures of N,N-Dimethylformamide with Some Dipolar Aprotic and Aprotic Solvents by Dielectric Characterization, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2009, 48, 512 Search PubMed
.
- C. Wang, H. Li, L. Zhu and S. Han, NMR and Excess Volumes Studies in DMF–Alcohol Mixtures, J. Solution Chem., 2002, 31, 109 CrossRef CAS
.
- Y. Lei, H. Li, H. Pan and S. Han, Structures and Hydrogen Bonding Analysis of N,N-Dimethylformamide and N,N-Dimethylformamide–Water Mixtures by Molecular Dynamics Simulations, J. Phys. Chem. A, 2003, 107, 1574 CrossRef CAS
.
- A. N. Kannappan, S. Thirumaran and R. Palani, Volumetric and Thermodynamic Studies of Molecular Interactions in Ternary Liquid Mixtures at 303, 308 and 313 K, J. Phys. Sci., 2009, 20, 97 CAS
.
- C. Hao, Y. Zhao, Y. Zhou, L. Zhou, Y. Xu, D. Wang and D. Xu, Interactions Between Metal Chlorides and Poly(Vinyl Pyrrolidone) in Concentrated Solutions and Solid-State Films, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 1589 CrossRef CAS
.
- P. Das, A. Kedia, P. S. Kumar, N. Large and T. K. Chini, Local Electron Beam Excitation and Substrate Effect on the Plasmonic Response of Single Gold Nanostars, Nanotechnology, 2013, 24, 405704 CrossRef PubMed
.
- A. J. Haes, C. L. Haynes, A. D. McFarland, G. C. Schatz, R. P. Van Duyne and S. Zou, Plasmonic Materials for Surface-Enhanced Sensing and Spectroscopy, MRS Bull., 2005, 30, 368 CrossRef CAS
.
- S. Nie and S. R. Emory, Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering, Science, 1997, 275, 1102 CrossRef CAS PubMed
.
- M. Fan, G. F. S. Andradec and A. G. Brolod, A Review on the Fabrication of Substrates for Surface Enhanced Raman Spectroscopy and their Applications in Analytical Chemistry, Anal. Chim. Acta, 2011, 693, 7 CrossRef CAS PubMed
.
- S. Yao, C. Zhou and D. Chen, Highly Porous PVA Dried Gel with Gold Nanoparticles Embedded in the Network as a Stable and Ultrasensitive SERS Substrate, Chem. Commun., 2013, 49, 6409 RSC
.
- S. Mura, G. Greppi, P. Innocenzi, M. Piccinini, C. Figus, M. L. Marongiu, C. Guob and J. Irudayaraj, Nanostructured Thin Films as Surface-Enhanced Raman Scattering Substrates, J. Raman Spectrosc., 2013, 44, 35 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: SERS spectra of pure and conjugated crystal violet on the gold nanoparticle surface along with the enhancement factor calculations are illustrated. See DOI: 10.1039/c4ra12846j |
| This journal is © The Royal Society of Chemistry 2015 |