Facile synthesis of single crystalline sub-micron Cu2ZnSnS4 (CZTS) powders using solvothermal treatment†
Abstract
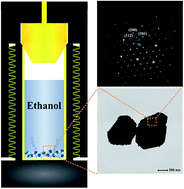
Single crystalline sub-micron Cu2ZnSnS4 (CZTS) powders were successfully synthesized by a facile solvothermal method, using L-cysteine as sulfur precursor. It was confirmed that pure kesterite structured CZTS powders were synthesized at 400 °C after 5 h, with the irregular polygonal particle size being 500 nm to 1 μm. Interestingly, single crystalline CZTS particles were also obtained by simple solvothermal treatment without post annealing. The H2S released from L-cysteine had an impact on the growth of CZTS particles at high temperature. Further, the as-synthesized CZTS powders were used as counter electrode for dye-sensitized solar cells (DSSCs) and it is indicated that the CZTS counter electrode based DSSCs show a conversion efficiency of 4.243%.

 Please wait while we load your content...
Please wait while we load your content...