Amine- and blocked isocyanate-terminated polyurethane dendrimers: integrated synthesis, photophysical properties and application in a heat curable system†
S.
Veerapandian
and
A. Sultan
Nasar
*
Department of polymer Science, University of Madras, Guindy Campus, Chennai 600 025, India. E-mail: drasultannasar@yahoo.com; drasultannasar@unom.ac.in; Tel: +044-22202820
First published on 4th December 2014
Abstract
A highly efficient, scalable, three step divergent synthesis of blocked isocyanate-terminated polyurethane dendrimers up to fourth generation (G4) has been developed avoiding the formation of free isocyanate. Amine-terminated polyurethane dendrimers were obtained as complementary functional materials in the second step. The blocked isocyanate moiety possessing easily cleavable blocking agents facilitated the dendrimer construction. Heat-curable adhesives based on G2 dendrimers tested on aluminum plates showed tensile shear strength of 2.41 MPa.
Introduction
Overcoming their laborious synthesis, dendrimers have emerged as a very important class of organic and polymeric materials in the last two decades.1 Although some research on dendrimers has been carried out, quantitative synthesis of dendrimers with less synthetic effort is still attractive.2 The globular shape of dendrimers provided a large surface area that can be decorated with different functional groups and chromophore for different applications; the application area includes semiconductor,3 molecular lens,4 organic light emitting diodes (OLEDs),2b,5 catalyst,6 crosslinking processe7 and variety of biological applications.8 Amine-terminated dendrimers are used as encapsulating agents for variety of drug molecules and such dendrimer-encapsulated drug delivery systems are used to cure disease like cancer,9 hypertension,10 thromposis,11 pupil dialator,12 antibacterial,13 antiparasite14 and anti-inflammatory.15 Blocked polyisocynates are a class of raw material used to produce single-component polyurethane products like powder coatings, heat setting adhesives etc.16 in which completely arresting of reactivity of isocyanate group is inevitable.Polyurethanes are generally prepared by a hydrogen addition reaction of an isocyanate with an alcohol. There are many methods available for generation of isocyanate monomers,17 but all the methods are cumbersome and some of the methods are not quantitative. It is obvious that quantitative conversion of chosen functional group into desired group is extremely important in the construction of dendrimers, or otherwise, the molecules would show defects.
Though polyurethanes are an important class of materials, their dendritic (including hyperbranched) molecules have been studied very poorly.17b,18 There are only two reports17b,18f which deal with perfect polyurethane dendrimer up to third generation. The reason may lie in the fact that the creation of isocyanate functionality in quantitative yield at each generation growth is very difficult with existing methods. Moreover, isocyanates are highly sensitive to moisture and hence difficult to handle. In this article, we break this challenge by designing a highly efficient method by which isocyanate groups were generated in a direct way in the form of blocked isocyanate with easily cleavable blocking agent via a non-isocyanate route. This route mainly involved conversion of amine functional group into blocked isocyanate group using 4-chlorophenychloroformate and urethane interchange reaction between blocked isocyanate and hydroxy building block; use of 4-chlorophenychloroformate facilitated easy construction of dendrimers terminated with –NH2 and blocked –NCO groups up to fourth generation.
Result and discussion
Synthesis
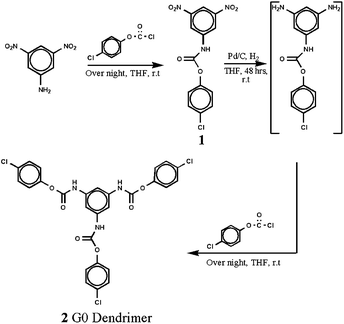
The facile synthesis of core was started with 3,5dinitroaniline. To obtain the core in a smooth way, the amine group was first converted into blocked isocyanate group by reacting it with 4-chlorophenyl chloroformate. Subsequent reduction and repetition of reaction with chloroformate yielded the symmetrical blocked triisocyanate core 2 with 98% yield (Scheme 1). Selection of phenyl chloroformate with chlorine substituent at 4-position is a vital key aspect in this design. The usual practice in the reaction of amine with chloroformate is the addition of mild base like pyridine or triethylamine to scavenge the HCl byproduct formed. Such approach was found to affect the yield too in the present work. The reason may be the activation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of chloroformate by the chlorine substituent in the benzene ring which leads to strong association of reagent with base. Hence, the availability of reagent for nucleophilic substitution reaction is reduced. Thus, this reaction was carried out by us without the addition of base. In this synthesis, the intermediate 1 and core 2 were purified simply by wash with petroleum ether.
O group of chloroformate by the chlorine substituent in the benzene ring which leads to strong association of reagent with base. Hence, the availability of reagent for nucleophilic substitution reaction is reduced. Thus, this reaction was carried out by us without the addition of base. In this synthesis, the intermediate 1 and core 2 were purified simply by wash with petroleum ether.
The structure of 1 and 2 was unambiguously confirmed using different spectroscopy techniques. In the 1H NMR spectrum, the core 2 showed a singlet at δ 10.4 for three urethane –NH protons in addition to signals for aromatic protons. In the 13C NMR spectrum, the urethane carbonyl carbon of compound 2 appeared at δ 151.3 in addition to six aromatic carbons. In the mass spectrum (HR-MS), the core 2 showed molecular ion peak at m/z equal to 584.9. The structure of the compound 2 was further confirmed from nitrogen content estimation.
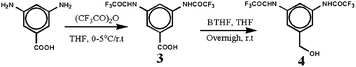
Building block (4) with protected amine at 3,5-position and benzylic –OH at 1-position was prepared from 3,5-diaminobenzoic acid in 2 steps as described in the Scheme 2. The intermediate 3 and building block 4 were extracted with ether and ethyl acetate respectively and were not subjected to column purification as they showed single spot in TLC; the overall yield was 98%. The structure of 3 and 4 was unambiguously confirmed using different spectroscopy techniques and nitrogen content estimation. In the 1H NMR spectrum, the building block 4 showed a singlet at δ 11.4, δ 5.4 and δ 4.5 for two amide –NH protons, one –OH protons and two aliphatic protons respectively in addition to two signals for aromatic protons. In the 13C NMR spectrum, the amide carbon and aliphatic carbon of compound 4 appeared at δ 154.5 and δ 62.4 respectively in addition to four aromatic carbons. In the mass spectrum (HR-MS), the building block 4 showed molecular ion peak at m/z equal to 329.53.
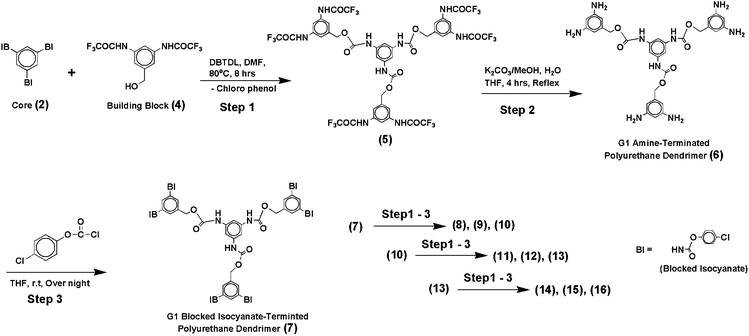
We designed a synthetic strategy adopting the concept of blocked isocyanate chemistry for the synthesis of polyurethane dendrimers. Blocked isocyanate is an adduct usually obtained from aromatic isocyanate and phenol. The –NHCOO– group of a blocked isocyanate is thermally weak and at elevated temperature it de-blocks into isocyanate (–NCO) and blocking agent (HO–). Then, the regenerated isocyanate could react with a co-reactant containing aliphatic hydroxyl functional group to form urethane with thermally more stable bonds.19 Dendrimer construction in the present work involved only three steps. In the first step, blocked triisocyanate core 2 (G0 dendrimer) was reacted with building block 4 containing hydroxyl group. The core was prepared in such a way that it contained 4-chlorophenol as blocking agent. The chlorine substituent at para-position to the urethane group will reduce the electron density significantly at the phenolic oxygen and labialize the bond between the nitrogen atom of the isocyanate moiety and oxygen atom of blocking agent moiety. This was reflected in the experiment, with the blocking agent cleaved off easily at 80 °C in DMF and hence urethane interchange reaction between core and building block proceeded smoothly; 90% of the reaction was completed within 2 hours and the conversion was fully completed within 8 hours. In the second step, the deprotection of amine at the periphery of the intermediate 5 obtained in 94% yield resulted in first generation amine-terminated urethane dendrimer 6. The condition employed for this conversion did not at all affect the existing urethane linkages and the yield was 95%. In the third step, the amine-terminated urethane dendrimer 6 was simply treated with 4-chlorophenyl chloroformate to obtain blocked isocyanate-terminated urethane dendrimer 7 in quantitative yield. It is worth mentioning here that, in this three step synthesis, we obtained two types of dendrimers (6,9,12 and 15) and (7,10,13 and 16) with utilizable two different functional groups and none of the compounds obtained in this reaction sequence required column purification; they were purified simply by re-precipitation in THF by addition of petroleum ether. Both types of dendrimers have potential applications in cross-linking processes especially in polyurethane products. This easy-to-conduct synthetic strategy is illustrated in the Scheme 3 (Fig. 1).
The 1H-NMR spectrum of G4 amine-terminated dendrimer 15 is given in Fig. 2 as a representative for 6, 9, 12 and 15. Since all the urethane linkages present in 15 are identical, the urethane protons have resonance only at δ 9.7. The large number of free –NH2 present as terminal groups have resonance strongly at δ 4.55. Because of the presence of free amines in the terminal ring, the adjacent aromatic protons of two different types appear separately at high field with proportional intensities. The integration values of different type of protons including urethane –NH and free amine agree well with the expected values and confirm the defect-free structure of the amine-terminated dendrimer.
 | ||
| Fig. 2 1H NMR Spectra of (a) amine-terminated and (b) blocked isocyanate-terminated G4 polyurethane dendrimer. | ||
The 1H-NMR spectrum of G4 blocked isocyanate-terminated dendrimer 16 is also given in Fig. 2 as a representative for 7, 10, 13 and 16. It shows sharp and distinctly separated signals for relatively large number of aliphatic, aromatic and urethane –NH protons. The free amine observed in the spectrum of previous case is completely disappeared here due to its complete conversion into blocked isocyanates groups. Unlike the case of amine terminated dendrimer 15, the –NH protons of terminal blocked isocyanate groups and inner urethane linkages of dendrimer 16 are not identical and appear separately at δ 10.4 and δ 9.8 respectively.
The MALDI-TOF MS spectrum of the same dendrimer showed only one peak with 100% intensity at m/z value of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952.5 which is exactly corresponding to [M + Na]+ (Fig. 3). The excellent agreement between the integration values of different types of protons (including –NH protons) observed in 1H-NMR spectrum and absence of any peaks in MALDI-TOF MS spectrum corresponding to incompletely converted products during the iterative process unambiguously confirm the structure and high purity of the dendrimer obtained in quantitative yield. Analysis of 1H-NMR spectra and MALDI-TOF MS spectra of other dendrimers reveals that those dendrimers [i.e., G1(7), G2(10) and G3(13)] are also structurally perfect and highly pure.
952.5 which is exactly corresponding to [M + Na]+ (Fig. 3). The excellent agreement between the integration values of different types of protons (including –NH protons) observed in 1H-NMR spectrum and absence of any peaks in MALDI-TOF MS spectrum corresponding to incompletely converted products during the iterative process unambiguously confirm the structure and high purity of the dendrimer obtained in quantitative yield. Analysis of 1H-NMR spectra and MALDI-TOF MS spectra of other dendrimers reveals that those dendrimers [i.e., G1(7), G2(10) and G3(13)] are also structurally perfect and highly pure.
SEC-MALLS analyses
As we obtained remarkably good MALDI-TOF MS spectra which confirm the defect free structure of dendrimers, it was decided to support these data. Size exclusion chromatography combined with multi angle laser light scattering (SEC-MALLS) technique is an ultimate tool to be used to determined absolute molar mass and polydispersity index (PDI) of the dendrimers. Thus, all the blocked isocyanate-terminated dendrimers were analyzed using SEC-MALLS and the results are given in Fig. 4 and Table 1. All the SEC traces are unimodel and molecular weight (Mn) data are also found comparable to the theoretical values. The unimodel chromatogram with narrow distribution clearly supports the MALDI-TOF MS spectra of the dendrimers.| Dendrimers | Theoretical molecular weight (g mol−1) | SEC-MALLS molecular weight (Mn) (g mol−1) | PDI |
|---|---|---|---|
| a dn/dc = 0.161. b dn/dc = 0.159. c dn/dc = 0.161. d dn/dc = 0.161. | |||
| G1 dendrimer (7) | 1543 | 2274a | 1.03 |
| G2 dendrimer (10) | 3455 | 3524b | 1.22 |
| G3 dendrimer (13) | 7280 | 6576c | 1.03 |
| G4 dendrimer (16) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929 929 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610d 610d |
1.00 |
Photoluminescence study
The absorption and emission spectra of dendrimers are given in Fig. 5. The intensity of absorption and emission become strong as the number of generation increased. This generation dependent photoluminescence reflects the highly globular geometry of higher generation dendrimer and enhanced energy transfer with in the molecule. Similar hyperchromic effect on poly(aryl-ether-urea) dendrimers has been reported from our laboratory.20 | ||
| Fig. 5 UV-visible and fluorescence spectra of blocked isocyanate-terminated polyurethane dendrimers. | ||
In addition, λmax (abs) and λmax (flu) were also increased (oxochromic) as the generation number increased, however the increment is found to be low. The absorption and emission bands were broad and without any vibrational structure. This is attributed to the decreased planarity of the molecules due to the large steric interaction with in the molecule.21 All the dendrimer showed strong visible fluorescence in the 524 nm to 535 nm range on excitation with 350 nm light or 470 nm light because of the presence of strongly absorbing phenyl rings (Table 2). A significantly high Stokes shift of 67 nm to 72 nm was absorbed between absorption and emission maxima. These results confirm the better performance of larger dendrimers as antennae to receive photons.
Solubility
Solubility of the dendrimers was determined by dissolving the compound (5 mg mL−1) in different highly polar and relatively less polar solvents. All the blocked isocyanate-terminated dendrimers were found highly soluble in solvents like DMF and NMP and found partially soluble in THF. Compared to THF, solubility of these dendrimers was poor in acetone and very poor in ethyl acetate. It was found that solubility was increased with increasing the generation number. Also, it was found that when compared to blocked isocyanate dendrimers, solubility of amine-terminated dendrimers was less in these solvents.Heat-cured adhesives
Since both type of dendrimers reported in this work possess reactive end functional groups, they could be used as potential candidates, more particularly, in crosslinking process. In this study, heat-setting single-component polyurethane adhesives based on G2 amine- and blocked isocyanate-terminated dendrimers were formulated and tested on aluminum plates (Fig. 6, Table 3). During the curing process, urethane linkages will be formed in the case of adhesive formulated with blocked OCN– dendrimer. Whereas in the case of Adhesive-2, urea linkages will be formed between H2N-dendrimer and blocked polyisocyanate in which urethane linkages are already present. The adhesive based on blocked isocyanate dendrimer was found to withstand tensile shear strength of 2.41 MPs. This values is sufficient to join and seal aluminum and ceramic parts, thus, valuable heat-curable single component adhesive-sealant could be produced using this formulation. The low adhesive strength of formulation based on amine-terminated dendrimer (Adhesive-2) is due to use of N-methylaniline blocked polyisocyanate as co-reactant in which the blocking agent could not be cleaved off as easily as 4-chlorophenol since the deblocking temperature of former is close to unsubstituted phenol.19 Indeed, it is possible to improve the performance of Adhesive-2 even better than the Adhesive-1 by changing the blocked polyisocyanate used. | ||
| Fig. 6 Tensile Shear test profile of heat-cured adhesive based on G2 blocked isocyanate-terminated dendrimer. | ||
| Adhesive no. | Ingredients (g) | Tensile shear strength (MPa) | |
|---|---|---|---|
| a Equivalent weight 1000 g mol−1. b Bonding agent. c N-Methylaniline blocked polyisocyanate (eq. wt 1421 g mol−1). | |||
| 1 | G2-blocked isocyanate-terminated dendrimer | 0.456 | 2.41 |
| Poly(polytetrahydrofuran carbonate) diola | 1.520 | ||
| Dibutyltin dilaurate | 0.004 | ||
| Silquest A 187b | 0.020 | ||
| 2 | G2-amine-terminated dendrimer | 0.176 | 0.93 |
| Blocked polyisocyanatec | 1.800 | ||
| Dibutyltin dilaurate | 0.004 | ||
| Silquest A 187b | 0.020 | ||
Conclusions
In conclusion, a simple and highly efficient method for quantitative synthesis of amine- and blocked isocyanate-terminated polyurethane dendrimers up to fourth generation has been developed. Purification of core, building block and dendrimers required only washing or re-precipitation. The defect-free structures of dendrimers were confirmed using 1H NMR and MALDI-TOF MS spectra and SEC-MALLS. All the dendrimer showed strong visible fluorescence in the 524 nm to 535 nm range. Heat-setting adhesive based on blocked isocyanate-terminated G2 dendrimer formulated for this study shown tensile shear strength of 2.41 MPa. This value is sufficient to join and seal aluminum and ceramic parts, thus, the dendrimers reported herein could be scale-up for new product development.Experimental
Materials
3,5-Dinitroaniline (TCI), 4-chlorophenyl chlorformte (Alfa aesar), 3,5-diaminobenzoic acid (Sigma-Aldrich), trifluoroacetic anhydride (Alfa aesar), dibutyltin dilaurate (Sigma-Aldrich) and 1 M borane–THF (Acros) were used as received. Dimethylformamide (Merck) and tetrahydrofuran (Merck) were purified using standard procedures.Measurements
FT-IR spectra were reordered by the KBr pellet method on a ABB MB3000 instrument. UV-visible spectra were recorded on Techcomp, UV2301 II spectrophotometer. Fluorescence spectra were recorded on HORIBA JOBINYVON Fluoromax-4 spectrofluorometer. High resolution mass spectra were recorded using a Q-TOF-Mass Spectrometer. NMR spectra were recorded on a Bruker AV 500 (1H NMR: 500 MHz; 13C NMR 125 MHz) or a Bruker AV 400 (1H NMR: 400 MHz; 13C NMR 100 MHz) or a Bruker AV 300 (1H NMR: 300 MHz; 13C NMR 75 MHz). Chemical shift values (δ) were given in ppm and were calibrated on residual non-deutrated solvent peaks (CDCl3: 1H NMR: 7.26 ppm, 13C NMR: 77.0 ppm; DMSO-d6: 1H NMR: 3.43, 2.54 ppm, 13C NMR: 39.5 ppm) as internal standard. MALDI-TOF MS spectra were recorded by positive mode on a Bruker Ultra flex instrument using dithranol as matrices. The absolute molecular weight of dendrimers were determined using SEC-MALLS at 60 °C (SEC: Shimadzu LC-20AD, Shimadu DGU-20 A3R and two numbers of PL gel 5 µm MIXED-C column. MALLS: Wyatt mini DAWN TREOS and Wyatt Optilab rEx). DMF containing 0.1mol L−1 of lithium bromide was used as eluents. Specific refractive index increment (dn/dc) for each dendrimers was determined separately in the same solvent used for SEC experiments and used for molecular weight calculation. The nitrogen content of the compounds was estimated using KELPLUS (Pelicon) nitrogen estimation system.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1594 (C–O–Ar). 1H NMR (400 MHz, DMSO-d6): δ 11.23 (s, 1H), 8.71 (s, 2H), 8.50 (s, 1H), 7.52 (d, J = 8.12, 2H), 7.35 (d, J = 8.64, 2H). 13C NMR (100 MHz, DMSO-d6): δ 151.46, 148.83, 148.38, 141.02, 130.15, 129.47, 123.74, 117.82, 112.17. Calcd: [M]+ m/z = 337.67. Found: HR-MS = 337.08. Nitrogen content for C13H8ClN3O6: calcd: 12.44%; found: 12.12%.
O), 1594 (C–O–Ar). 1H NMR (400 MHz, DMSO-d6): δ 11.23 (s, 1H), 8.71 (s, 2H), 8.50 (s, 1H), 7.52 (d, J = 8.12, 2H), 7.35 (d, J = 8.64, 2H). 13C NMR (100 MHz, DMSO-d6): δ 151.46, 148.83, 148.38, 141.02, 130.15, 129.47, 123.74, 117.82, 112.17. Calcd: [M]+ m/z = 337.67. Found: HR-MS = 337.08. Nitrogen content for C13H8ClN3O6: calcd: 12.44%; found: 12.12%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Carbamate), 1201 (C–O–Ar). 1H NMR (500 MHz, DMSO-d6): δ 10.39 (s, 3H), 7.53–7.29 (ArH, 15H), 13C NMR (125 MHz, DMSO-d6): δ 151.27, 149.27, 139.23, 129.51, 129.26, 123.83. Calcd: [M]+ m/z = 586.81. Found: HR-MS = 584.91. Nitrogen content for C27H18Cl3N3O6: calcd: 7.16%; found: 6.94%.
O Carbamate), 1201 (C–O–Ar). 1H NMR (500 MHz, DMSO-d6): δ 10.39 (s, 3H), 7.53–7.29 (ArH, 15H), 13C NMR (125 MHz, DMSO-d6): δ 151.27, 149.27, 139.23, 129.51, 129.26, 123.83. Calcd: [M]+ m/z = 586.81. Found: HR-MS = 584.91. Nitrogen content for C27H18Cl3N3O6: calcd: 7.16%; found: 6.94%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620 (acid –OH), 1205 (–CF3). 1H NMR (400 MHz, DMSO-d6): δ 11.57 (s, 2H), 8.43 (s, 1H), 8.14 (s, 2H), 13C NMR (100 MHz, DMSO-d6): δ 166.19, 154.92, 154.55, 137.14, 132.11, 118.58, 114.13. Calcd: [M]+ m/z = 344.17. Found: HR-MS = 344.02. Nitrogen content for C11H6F6N2O4: calcd: 8.14%; found: 7.98%.
O), 1620 (acid –OH), 1205 (–CF3). 1H NMR (400 MHz, DMSO-d6): δ 11.57 (s, 2H), 8.43 (s, 1H), 8.14 (s, 2H), 13C NMR (100 MHz, DMSO-d6): δ 166.19, 154.92, 154.55, 137.14, 132.11, 118.58, 114.13. Calcd: [M]+ m/z = 344.17. Found: HR-MS = 344.02. Nitrogen content for C11H6F6N2O4: calcd: 8.14%; found: 7.98%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620.1H NMR (500 MHz, DMSO-d6): δ 11.38 (s. 2H), 8.14 (s, 1H), 7.66 (s, 2H), 5.39 (t, 1H), 4.35 (dd, J = 5, 2H), 13C NMR (125 MHz, DMSO-d6): 154.74, 139.62, 136.65, 116.90, 115.99, 112.02, 62.42. Calcd: [M]+ m/z = 330.18. Found: HR-MS = 329.53. Nitrogen content for C11H8F6N2O3: calcd: 8.48%; found: 8.21%.
O), 1620.1H NMR (500 MHz, DMSO-d6): δ 11.38 (s. 2H), 8.14 (s, 1H), 7.66 (s, 2H), 5.39 (t, 1H), 4.35 (dd, J = 5, 2H), 13C NMR (125 MHz, DMSO-d6): 154.74, 139.62, 136.65, 116.90, 115.99, 112.02, 62.42. Calcd: [M]+ m/z = 330.18. Found: HR-MS = 329.53. Nitrogen content for C11H8F6N2O3: calcd: 8.48%; found: 8.21%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1564 (C–O–R), 1206 (–CF3) 1H NMR (500 MHz, DMSO-d6): δ 11.52 (s, 6H), 9.86 (d, J = 14.7, 3H), 8.18 (s, 3H), 7.60–7.34 (m, 9H), 5.19 (s, 6H), 13C NMR (75 MHz, DMSO-d6): 154.81, 154.32, 142.30, 136.77, 134.99, 129.95, 115.49, 113.76, 112.31, 64.65. Calcd: [M]+ m/z = 1191.15. Found: HR-MS = 1191.81. Nitrogen content for C42H27F18N9O12: calcd: 10.58%; found: 10.41%.
O), 1564 (C–O–R), 1206 (–CF3) 1H NMR (500 MHz, DMSO-d6): δ 11.52 (s, 6H), 9.86 (d, J = 14.7, 3H), 8.18 (s, 3H), 7.60–7.34 (m, 9H), 5.19 (s, 6H), 13C NMR (75 MHz, DMSO-d6): 154.81, 154.32, 142.30, 136.77, 134.99, 129.95, 115.49, 113.76, 112.31, 64.65. Calcd: [M]+ m/z = 1191.15. Found: HR-MS = 1191.81. Nitrogen content for C42H27F18N9O12: calcd: 10.58%; found: 10.41%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1564 (C–OR), 1206 (–CF3). 1H –NMR (500 MHz, DMSO-d6): δ 11.52 (s, 12H), 9.89 (s, 9H), 8.18 (s, 12H), 7.60 (s, 12H), 7.46–7.34 (m, 6H), 5.19 (s, 18H), 13C NMR (75 MHz, DMSO-d6): 155.29, 154.80, 154.32, 153.82, 136.61, 134.48, 129.16, 127.49, 117.64, 116.08, 113.81, 112.83, 62.40.
O), 1564 (C–OR), 1206 (–CF3). 1H –NMR (500 MHz, DMSO-d6): δ 11.52 (s, 12H), 9.89 (s, 9H), 8.18 (s, 12H), 7.60 (s, 12H), 7.46–7.34 (m, 6H), 5.19 (s, 18H), 13C NMR (75 MHz, DMSO-d6): 155.29, 154.80, 154.32, 153.82, 136.61, 134.48, 129.16, 127.49, 117.64, 116.08, 113.81, 112.83, 62.40.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1564 (C–O–R), 1206 (–CF3). 1H NMR (500 MHz, DMSO-d6): δ 11.48 (s, 24H), 9.76 (s, 21H), 8.74 (d, 24H, J = 2.4), 8.53 (t, 12H, J = 2.8), 7.65–7.35 (m, 30H), 6.72 (s, 42H), 13C NMR (75 MHz, DMSO-d6): 163.35, 147.92, 141.16, 139.22, 127.52, 126.12, 117.71, 116.63, 102.14, 101.26, 63.66.
O), 1564 (C–O–R), 1206 (–CF3). 1H NMR (500 MHz, DMSO-d6): δ 11.48 (s, 24H), 9.76 (s, 21H), 8.74 (d, 24H, J = 2.4), 8.53 (t, 12H, J = 2.8), 7.65–7.35 (m, 30H), 6.72 (s, 42H), 13C NMR (75 MHz, DMSO-d6): 163.35, 147.92, 141.16, 139.22, 127.52, 126.12, 117.71, 116.63, 102.14, 101.26, 63.66.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1564 (C–O–R), 1206 (–CF3). 1H NMR (500 MHz, DMSO-d6): δ 11.48 (s, 48H), 9.76 (s, 45H), 8.74 (s, 48H), 8.53 (s, 124H), 7.65–7.35 (m, 66H), 6.72 (m, 90H), 13C NMR (75 MHz, DMSO-d6): 163.65, 147.92, 141.16, 139.22, 127.52, 126.12, 117.71, 116.63, 102.14, 101.26, 63.66.
O), 1564 (C–O–R), 1206 (–CF3). 1H NMR (500 MHz, DMSO-d6): δ 11.48 (s, 48H), 9.76 (s, 45H), 8.74 (s, 48H), 8.53 (s, 124H), 7.65–7.35 (m, 66H), 6.72 (m, 90H), 13C NMR (75 MHz, DMSO-d6): 163.65, 147.92, 141.16, 139.22, 127.52, 126.12, 117.71, 116.63, 102.14, 101.26, 63.66.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water–methanol. The reaction mixture was reflexed for 4 h. After completion of the reaction, the reaction mixture was concentrated under vacuum at 50 °C. The resultant paste was washed repeatedly with water to yield fine product in brown color. Yield (91.6%), M.p: 186–188 °C, IR (KBr, cm−1) 3371 (–NH2 stretching), 1713 (C
1 mixture of water–methanol. The reaction mixture was reflexed for 4 h. After completion of the reaction, the reaction mixture was concentrated under vacuum at 50 °C. The resultant paste was washed repeatedly with water to yield fine product in brown color. Yield (91.6%), M.p: 186–188 °C, IR (KBr, cm−1) 3371 (–NH2 stretching), 1713 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C–O–R). 1H NMR (300 MHz, DMSO-d6): δ 9.63 (s, 3H), 7.33 (s, 3H), 5.82 (s, 6H), 5.77 (s, 3H), 4.83 (s, 6H), 4.75 (s, 12H), 13C NMR (75 MHz, DMSO-d6): 153.63, 139.49, 138.16, 136.00, 117.14, 116.49, 114.43, 113.07, 64.84. Nitrogen content for C30H33N9O6: calcd: 20.48%; found: 20.18%.
O), 1589 (C–O–R). 1H NMR (300 MHz, DMSO-d6): δ 9.63 (s, 3H), 7.33 (s, 3H), 5.82 (s, 6H), 5.77 (s, 3H), 4.83 (s, 6H), 4.75 (s, 12H), 13C NMR (75 MHz, DMSO-d6): 153.63, 139.49, 138.16, 136.00, 117.14, 116.49, 114.43, 113.07, 64.84. Nitrogen content for C30H33N9O6: calcd: 20.48%; found: 20.18%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C–O–R) 1H NMR (500 MHz, DMSO-d6): δ 6.63 (s, 6H), 7.33 (s, 3H), 7.04 (s, 3H), 7.14 (s, 6H), 5.82 (s, 12H), 5.77 (S, 6H), 5.19 (s, 18H), 5.25 (s, 24H), 13C NMR (75 MHz, DMSO-d6): 154.81, 154.32, 136.77, 134.99, 132.63, 129.95, 127.94, 117.58, 115.49, 113.76, 112.31, 64.65.
O), 1589 (C–O–R) 1H NMR (500 MHz, DMSO-d6): δ 6.63 (s, 6H), 7.33 (s, 3H), 7.04 (s, 3H), 7.14 (s, 6H), 5.82 (s, 12H), 5.77 (S, 6H), 5.19 (s, 18H), 5.25 (s, 24H), 13C NMR (75 MHz, DMSO-d6): 154.81, 154.32, 136.77, 134.99, 132.63, 129.95, 127.94, 117.58, 115.49, 113.76, 112.31, 64.65.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 9.68 (s, 21H), 7.52–7.49 (m, 9H), 7.38–7.30 (m, 21H), 5.75 (s, 24H), 5.49 (s, 12H), 4.99 (s, 42H), 4.56 (s, 48H), 13C NMR (75 MHz, DMSO-d6): 154.30, 136.72, 134.92, 132.61, 129.93, 127.90, 117.62, 115.44, 113.72, 112.29, 64.62.
O), 1589 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 9.68 (s, 21H), 7.52–7.49 (m, 9H), 7.38–7.30 (m, 21H), 5.75 (s, 24H), 5.49 (s, 12H), 4.99 (s, 42H), 4.56 (s, 48H), 13C NMR (75 MHz, DMSO-d6): 154.30, 136.72, 134.92, 132.61, 129.93, 127.90, 117.62, 115.44, 113.72, 112.29, 64.62.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 9.68 (s, 45H), 7.50 (s, 21H), 7.33 (s, 42H), 5.75 (s, 48H), 5.49 (s, 24H), 4.99 (s, 90H), 4.56 (s, 96H), 13C NMR (75 MHz, DMSO-d6): 154.36, 136.78, 134.96, 132.62, 129.91, 127.86, 117.64, 115.40, 113.76, 112.31, 64.66.
O), 1589 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 9.68 (s, 45H), 7.50 (s, 21H), 7.33 (s, 42H), 5.75 (s, 48H), 5.49 (s, 24H), 4.99 (s, 90H), 4.56 (s, 96H), 13C NMR (75 MHz, DMSO-d6): 154.36, 136.78, 134.96, 132.62, 129.91, 127.86, 117.64, 115.40, 113.76, 112.31, 64.66.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620 (C–O–R). 1H NMR (400 MHz, DMSO-d6): δ 10.36 (s, 6H), 9.73 (s, 3H), 7.45–7.22 (m, 27H), 6.77 (s, 6H), 5.05 (s, 6H), 13C NMR (75 MHz, DMSO-d6): 156.28, 151.26, 149.28, 139.23, 129.50, 129.26, 129.08, 123.82, 122.29, 116.88, 104.47, 67.47. Calcd: [M + Na]+ m/z = 1565.1. Found: MALDI-TOF-MS [M + Na]+ = 1564.4. Nitrogen content for C72H51Cl6N9O18: calcd: 8.17%; found: 8.04%.
O), 1620 (C–O–R). 1H NMR (400 MHz, DMSO-d6): δ 10.36 (s, 6H), 9.73 (s, 3H), 7.45–7.22 (m, 27H), 6.77 (s, 6H), 5.05 (s, 6H), 13C NMR (75 MHz, DMSO-d6): 156.28, 151.26, 149.28, 139.23, 129.50, 129.26, 129.08, 123.82, 122.29, 116.88, 104.47, 67.47. Calcd: [M + Na]+ m/z = 1565.1. Found: MALDI-TOF-MS [M + Na]+ = 1564.4. Nitrogen content for C72H51Cl6N9O18: calcd: 8.17%; found: 8.04%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1558 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 10.36 (s, 12H), 9.73 (s, 9H), 9.74 (s, 9H) 7.74–7.42 (m, 24H), 7.32 (s, 3H), 6.77–6.75 (m, 18H), 5.05 (s, 18H), 13C NMR (125 MHz, DMSO-d6): 154.77, 154.47, 153.03, 139.60, 139.50, 138.74, 136.93, 121.91, 117.16, 116.79, 114.49, 113.00, 64.84. Calcd: [M + Na]+ m/z = 1565.1. Found: MALDI-TOF-MS [M + Na]+ = 3477.9.
O), 1558 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 10.36 (s, 12H), 9.73 (s, 9H), 9.74 (s, 9H) 7.74–7.42 (m, 24H), 7.32 (s, 3H), 6.77–6.75 (m, 18H), 5.05 (s, 18H), 13C NMR (125 MHz, DMSO-d6): 154.77, 154.47, 153.03, 139.60, 139.50, 138.74, 136.93, 121.91, 117.16, 116.79, 114.49, 113.00, 64.84. Calcd: [M + Na]+ m/z = 1565.1. Found: MALDI-TOF-MS [M + Na]+ = 3477.9.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1558 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 10.44 (s, 24H), 9.66 (s, 18H), 8.11 (s, 21H), 7.72–7.46 (m, 99H), 6.70 (s, 42H), 4.81 (s, 48H), 13C NMR (75 MHz, DMSO-d6): 156.22, 151.16, 149.27, 139.21, 129.53, 129.26, 129.10, 123.84, 122.27, 116.90, 104.44, 67.48. Calcd: [M + Na]+ m/z = 7302.1. Found: MALDI-TOF-MS [M + Na]+ = 7302.3.
O), 1558 (C–O–R). 1H NMR (500 MHz, DMSO-d6): δ 10.44 (s, 24H), 9.66 (s, 18H), 8.11 (s, 21H), 7.72–7.46 (m, 99H), 6.70 (s, 42H), 4.81 (s, 48H), 13C NMR (75 MHz, DMSO-d6): 156.22, 151.16, 149.27, 139.21, 129.53, 129.26, 129.10, 123.84, 122.27, 116.90, 104.44, 67.48. Calcd: [M + Na]+ m/z = 7302.1. Found: MALDI-TOF-MS [M + Na]+ = 7302.3.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1558 (C–O–R). 1H NMR (400 MHz, DMSO-d6): δ 10.44 (s, 48H), 9.97 (s, 45H), 8.11 (s, 45H), 7.72–7.46 (m, 195H), 6.70 (s, 90H), 4.81 (s, 90H), 13C NMR (75 MHz, DMSO-d6): 156.22, 151.31, 149.26, 139.21, 129.52, 129.24, 129.09, 123.84, 122.27, 116.90, 104.46, 67.48. Calcd: [M + Na]+ m/z = 14
O), 1558 (C–O–R). 1H NMR (400 MHz, DMSO-d6): δ 10.44 (s, 48H), 9.97 (s, 45H), 8.11 (s, 45H), 7.72–7.46 (m, 195H), 6.70 (s, 90H), 4.81 (s, 90H), 13C NMR (75 MHz, DMSO-d6): 156.22, 151.31, 149.26, 139.21, 129.52, 129.24, 129.09, 123.84, 122.27, 116.90, 104.46, 67.48. Calcd: [M + Na]+ m/z = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952.8. Found: MALDI-TOF-MS [M + Na]+ = 14
952.8. Found: MALDI-TOF-MS [M + Na]+ = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952.5.
952.5.
Preparation and testing of polyurethane adhesive
A heat-setting polyurethane adhesive formulation containing the ingredients as mentioned in Table 3 was prepared in a 50 mL flask. To make this formulation as a homogenous mixture, initially all the ingredients were dissolved in 10 of THF and then the solvent was removed using rotatory evaporator at 35 °C. The adhesive paste was applied on surface cleaned aluminum plates with application area 22 mm × 23 mm and then cured in an oven maintained at 125 °C for 6 h and post-cured at 80 °C for 8 h. The tensile shear strength of the adhesive was tested on an UTM (Instron) with cross-head speed of 100 mm min−1. Three tests were performed for an average value.Acknowledgements
The authors thank the Department of Science of Technology, Government of India for financial support for this work (SERB Sanction Order no. and Date: SR/S1/PC-08/2010 (G).Dt. (13-07-2011).Notes and references
- (a) J. M. J. Frechet and D. A. Tomalia, Dendrimers and other Dendritic Polymers, Wiley, Chichester, UK, 2001 Search PubMed; (b) A. M. Caminade, C. O. Turrin, R. Laurent, A. Ouali and B. Delavaux-Nicot, Dendrimers. Towards Catalytic, Material and Bio-medical Uses, Wiley, Chichester, UK, 2011 Search PubMed; (c) M. Malkoch, E. M. almstrom and A. M. Aystrom, Polymer Science: A Comprehensive Reference, ed. A. H. E. Muller and K. L. Wooly, Elsevier, 2012, vol. 6, pp. 113–176 Search PubMed.
- (a) S. Xiao, N. Fu, K. Peckham and B. D. Smith, Org. Lett., 2010, 12, 140 CrossRef CAS PubMed; (b) E. J. Wren, X. Wang, A. Farlow, L. Shin-Chun, P. L. Burn and P. Meredith, Org. Lett., 2010, 12, 4338 CrossRef CAS PubMed; (c) R. B. Restani, P. I. Morgado, M. P. Riberiro, I. Correia, A. Aguiar-Ricardo and V. D. B. Bonifacio, Angew. Chem., Int. Ed., 2012, 51, 5162 CrossRef CAS PubMed.
- (a) P. L. Burn, S. C. Lo and I. D. W. Samuel, Adv. Mater., 2007, 19, 1675 CrossRef CAS; (b) M. E. Kose, W. J. Mitchell, N. Kopidakis, C. H. Chang, S. E. Shaheen, K. Kim and G. Rumbles, J. Am. Chem. Soc., 2007, 129, 14257 CrossRef PubMed.
- P. Furuta and J. M. J. Frechet, J. Am. Chem. Soc., 2003, 125, 13173 CrossRef CAS PubMed.
- M. Vanjinathan, H.-C. Lin and A. S. Nasar, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3806 CrossRef CAS.
- (a) T. Mu, D. Feng and A. Feng, J. Phys. Chem. C, 2008, 112, 5952 CrossRef CAS; (b) J. A. Johnson, J. J. Makis, K. A. Marvin, S. E. Rodenbusch and K. J. Stevenson, J. Phys. Chem. C, 2013, 117, 22644 CrossRef CAS.
- C. Sivakumar and A. S. Nasar, J. Appl. Polym. Sci., 2011, 120, 725 CrossRef CAS.
- C. C. Lee, J. A. MacKay, J. M. J. Ferchet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517 CrossRef CAS PubMed.
- (a) M. Mei, Y. Ren and X. Zhou, J. Appl. Polym. Sci., 2009, 114, 3760 CrossRef CAS; (b) G. F. Pan, Y. Lemmouchi, E. O. Akala and O. J. Bakare, J. Bioact. Compat. Polym., 2005, 20, 113 CrossRef CAS PubMed.
- B. Devarakonda, D. P. Otto and A. A. Judefeind, Int. J. Pharm., 2007, 345, 142 CrossRef CAS PubMed.
- (a) S. H. Bai, C. Thomas and F. Ahsan, J. Pharm. Sci., 2007, 339, 231 Search PubMed; (b) S. H. Bai and F. Ahsan, Pharm. Res., 2009, 26, 539 CrossRef CAS PubMed.
- T. F. Vandamme and L. Brobeck, J. Controlled Release, 2005, 102, 23 CrossRef CAS PubMed.
- M. L. Ma, Y. Y. Chang and Z. H. Xu, Eur. J. Med. Chem., 2007, 42, 93 CrossRef CAS PubMed.
- B. Devarakonda, R. A. Hill and W. Liebenberg, Int. J. Pharm., 2005, 304, 193 CrossRef CAS PubMed.
- (a) Y. Y. Cheng and T. W. Xu, Eur. J. Med. Chem., 2005, 40, 1188 CrossRef CAS PubMed; (b) Y. Y. Cheng, T. W. Xu and R. Q. Fu, Eur. J. Med. Chem., 2005, 40, 1390 CrossRef CAS PubMed; (c) N. Man, Y. Y. Cheng and T. W. Xu, Eur. J. Med. Chem., 2006, 41, 670 CrossRef CAS PubMed; (d) Y. Y. Cheng, N. Man and T. W. Xu, J. Pharm. Sci., 2007, 96, 595 CrossRef CAS PubMed; (e) W. J. Yang, Y. W. Li and Y. Y. Chang, J. Pharm. Sci., 2009, 98, 1075 CrossRef CAS PubMed.
- (a) D. A. wicks and Z. W. Wicks Jr, Prog. Org. Coat., 1999, 36, 148 CrossRef CAS; (b) D. A. wicks and Z. W. Wicks Jr, Prog. Org. Coat., 2001, 41, 1 CrossRef CAS.
- (a) H. Ulrich, Chemistry and Technology of Isocyanates, Wiley, Chichester, UK, 1996 Search PubMed; (b) R. T. Taylor and U. Puapaiboon, Tetrahedron Lett., 1998, 39, 8005 CrossRef CAS.
- (a) R. Spindler and J. M. J. Frechet, J. Chem. Soc., Perkin Trans. 1, 1993, 913 RSC; (b) A. Kumar and S. Ramakrishnan, J. Chem. Soc., Chem. Commun., 1993, 1453 RSC; (c) R. Spindler and J. M. J. Frechet, Macromolecules, 1993, 26, 4809 CrossRef CAS; (d) A. Kumar and S. Ramakrishnan, J. Polym. Sci., Part A: Polym. Chem., 1996, 34, 839 CrossRef CAS; (e) A. J. Clark, J. Echenique, D. M. Haddleton, T. A. Straw and P. C. Taylor, J. Org. Chem., 2001, 66, 8687 CrossRef CAS; (f) W. J. Feast, S. P. Rannard and A. Stoddart, Macromolecules, 2003, 36, 9704 CrossRef CAS; (g) C. Gao and D. Yan, Macromolecules, 2003, 36, 613 CrossRef CAS; (h) S. P. Rannard, N. J. Davis and I. Herbert, Macromolecules, 2004, 37, 9418 CrossRef CAS; (i) B. Bruchmann, Macromol. Mater. Eng., 2007, 292, 981 CrossRef CAS; (j) M. Vanjinathan, A. Shanavas, A. Raghavan and A. S. Nasar, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3877 CrossRef CAS; (k) M. Vanjinathan and A. S. Nasar, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 713 CrossRef CAS; (l) A. Stoddart, W. J. Feast and S. P. Rannard, Soft Matter, 2012, 8, 1096 RSC.
- G. Shankar and A. S. Nasar, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 15 Search PubMed.
- M. Vanjinathan and A. S. Nasar, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 713 CrossRef CAS.
- Y. R. Lai, X. Kong, S. A. Jenekhe and J. A. Bard, J. Am. Chem. Soc., 2003, 125, 12631 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of compound 2, 4–16 and isotopes distribution pattern (MALDI-TOF) of G1, G2, G3 and G4 dendrimers are available. See DOI: 10.1039/c4ra12751j |
| This journal is © The Royal Society of Chemistry 2015 |