Borohydride synthesis strategy to fabricate YBO3:Eu3+ nanophosphor with improved photoluminescence characteristics
Shubham Srivastavaa,
Aparna Mondalb,
Niroj Kumar Sahuc,
Shantanu K. Beheraa and
Bibhuti B. Nayak*a
aDepartment of Ceramic Engineering, National Institute of Technology Rourkela, Odisha-769008, India. E-mail: bbnayak@nitrkl.ac.in; bibhutib@gmail.com
bDepartment of Chemistry, National Institute of Technology Rourkela, Odisha-769008, India
cCenter for Nanotechnology Research, VIT University, Vellore-632014, India
First published on 5th January 2015
Abstract
This paper investigates for the first time the use of sodium borohydride (NaBH4) as a boron source as well as a precipitating agent for successfully preparing high temperature stable YBO3:Eu3+ nanophosphor. Moreover, the potential of this unique borohydride synthesis strategy is compared with the conventional boric acid based solid-state route in terms of phase stability, morphology and photoluminescence characteristics. The novel synthesis approach adopted here renders a high temperature stable phase pure YBO3:Eu3+ nanophosphor at 1200 °C with nearly spherical dumb-bell shaped particles in the range of ∼200 nm to 700 nm and exhibits improved color purity when compared to the irregular polyhedral shaped (size ∼5 μm) YBO3:Eu3+ phosphor prepared by the conventional solid-state method. Color purity, determined in terms of R/O ratio, of borohydride YBO3:Eu3+ nanophosphor was found to be 1.72 for the near vacuum ultra-violet (VUV is characterized with wavelength shorter than 200 nm) wavelength of 205 nm, which gradually decreases with increase in excitation wavelength. It has never been reported that the color of YBO3:Eu3+ phosphors can be tuned from pink to reddish-orange. The chromaticity coordinates revealed evidently that the YBO3:Eu3+ phosphor emission is tunable by simply adjusting the excitation wavelengths. In addition to providing spectroscopic data the reason for better color purity and tunability of chromaticity coordinates are also accredited in this work.
YBO3:Eu3+ phosphor has garnered immense focus owing to its strong photoluminescence intensity, high ultraviolet transparency and exceptional optical damage threshold as well as its prospective utilization in a myriad of applications spanning from flat display panels, white light emitting diodes to Hg free fluorescent lamps.1–3 Numerous synthesis methods such as sol–gel,4 solvothermal,5 co-precipitation,6,7 hydrothermal8,9 and spray pyrolysis10 have been developed for the formation of YBO3:Eu3+ phosphor. However, almost all of these syntheses have made use of boric acid (H3BO3) as a boron source. The use of tributyl borate (C12H27BO3) as a boron source has also been investigated.11 Nevertheless, the results are not better than those obtained by boric acid. A notable point is that when the stoichiometric ratio of boric acid is maintained, phase purity is hardly achieved. Even there have been reports on the use of excess boric acid (∼100% excess) to form phase pure YBO3.12 This demonstrates the difficulty in obtaining phase purity of the phosphor. An impurity phase Y3BO6, which develops at higher temperatures, has been accounted for a drastic decrease of the photoluminescence intensity.13 Besides, the color purity (which is generally assessed by R/O ratio) associated with YBO3:Eu3+ phosphor has been a cause of concern. The R/O ratio is strongly dependent on the particle size of the phosphor. Further, the particle size can be tuned to a smaller dimension by adopting a novel synthesis approach. Syntheses strategies employed for the phosphor preparation aim towards improving the color purity and chromaticity of YBO3:Eu3+ phosphor.14–16
Our approach allows accomplishing this goal by means of a unique synthesis strategy for the production of high temperature stable phase pure YBO3:Eu3+ along with an improvement in the color purity and chromaticity. A synthesis strategy based on the inclusion of sodium borohydride (NaBH4), both as a boron source as well as a precipitating agent, is reported here. To the best of our knowledge, there have been no reports on the room temperature fabrication of YBO3:Eu3+ phosphor using only NaBH4. In the work described in this paper the potential of an apparently innovative borohydride synthesis approach has been justified by carrying out a detailed analysis of phase stability, morphology, photoluminescence, color purity and chromaticity. This kind of sodium borohydride based controlled precipitation route reported here puts forth a new approach to attain the nano sized YBO3:Eu3+ phosphors. In addition, a comparison was drawn with regards to boric acid based conventional solid-state derived YBO3:Eu3+ phosphor.
Borohydride synthesis through precipitation technique was adopted to prepare 10 mol% Eu3+-doped YBO3 phosphor material. Analytical grade (99.9% pure) reagents of yttrium oxide (Y2O3) and europium oxide (Eu2O3) were used as the starting materials. Appropriate amounts of these two materials are dissolved in hydrochloric acid (HCl) to form a precursor solution. Borohydride reaction was conducted at room temperature with drop wise addition of appropriate amount of aqueous NaBH4 to the above precursor solution, with constant stirring using a magnetic stirrer. At a pH of ∼10, the precipitates were formed and then were washed several times with distilled water to remove unwanted products. After washing, the precipitate was dried and then calcined at 1200 °C for 1 h. For the sake of comparison, the conventional solid-state method was also employed to prepare the YBO3:Eu3+ phosphor with same concentration. The appropriate amount of Y2O3, Eu2O3 and H3BO3 were mixed properly and calcined at three different temperatures (500 °C, 1000 °C and 1200 °C for 1 h), with an intermediate grinding step followed after each heat treatment. Phase analysis was carried out using X-ray diffractometer (Rigaku Ultima-IV X-Ray Diffractometer) with Cu Kα radiation (λ = 1.5406 Å). The morphology of the prepared samples was studied by Field emission scanning electron microscopy (FESEM) (Nova Nano SEM/FEI 450). The luminescence spectra were taken for excitation wavelengths of 205 nm, 210 nm, 220 nm, 230 nm and 240 nm with the help of F-7000 FL spectrophotometer.
X-ray diffraction patterns of YBO3:Eu3+ phosphor materials prepared using borohydride and solid-state method are presented in Fig. 1.
 | ||
| Fig. 1 XRD patterns of calcined (1200 °C) YBO3:Eu3+ powders prepared using (a) conventional solid-state and (b) borohydride method. | ||
All the peaks of both XRD patterns are well indexed with phase pure YBO3, as per the JCPDS file number: 16-0277. It is notable to observe that the YBO3 phase, by the borohydride route, was stable at a high temperature of 1200 °C. Most prevalent Y3BO6 impurity phase was not observed up to 1200 °C, which is a significantly higher temperature than the earlier reports for the formation of the Y3BO6 impurity phase.13,17 The crystallite size of borohydride synthesized YBO3:Eu3+ phosphor was found to be ∼47 nm, which was smaller than the solid-state derived phosphor (62 nm).
FESEM micrographs of YBO3:Eu3+ phosphor materials prepared using solid-state and borohydride methods are shown in Fig. 2(a) and (b) respectively. In case of the solid-state route, clustering of polyhedral crystals form irregular shaped particles of size ∼5 μm. On the contrary, morphology of YBO3:Eu3+ particles prepared by borohydride route exhibits fine dumb-bell shaped particles which may have been formed due to high temperature induced coarsening of finer spherical crystallites. The coarsening rate is reasonably low, which is evident by only necking of spherical particles. Even after high temperature treatment, the particle aggregate is fairly minimal within a size in the range of 200 nm to 700 nm.
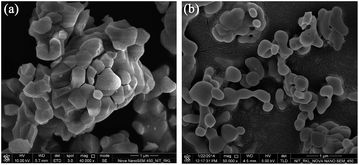 | ||
| Fig. 2 FESEM micrographs of calcined (1200 °C) YBO3:Eu3+ powders prepared using (a) conventional solid state and (b) borohydride method. | ||
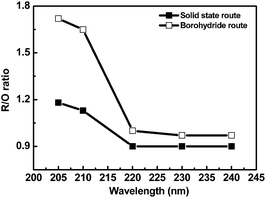
Emission spectra of YBO3:Eu3+ phosphor prepared using two methods are compared at an excitation wavelength of 205, 210, 220, 230 and 240 nm and is shown in Fig. 3. The major emissions are at 595 nm corresponding to 5D0 → 7F1 transition and 614 nm and 630 nm corresponding to 5D0 → 7F2 transition. The transition of 5D0 → 7F2 (red emission) is due to the typical electric dipole transition, and the transition of 5D0 → 7F1 (orange emission) is attributed to the magnetic dipole transition.1 It is very clear that the luminescence intensity of the phosphor particles corresponding to the solid-state method of preparation is high, which is in striking contrast with the behavior of the particles prepared using the borohydride route, that demonstrate lower luminescence intensity. This is ascribed to the size of the particles, which is towards the lower range in the nanometer scale for the phosphor particles of the borohydride route. For plasma display panels (PDPs), poor color purity hampers the use of this phosphor, which corresponds to the fact that the intensity of red emission of YBO3:Eu3+ is lower than that of the orange one.18 Color purity is denoted as R/O ratio [I(5D0 → 7F2)/I(5D0 → 7F1)] and is calculated by considering the sum of integral intensity of red emission peaks observed at 614 and 630 nm for contribution of 5D0 → 7F2 transition. From Fig. 3, it seems that at lower excitation wavelengths, the color purity of borohydride-derived phosphor is higher than the solid-state derived phosphor. This can be succinctly expressed by means of the variation of R/O ratio with the excitation wavelength as revealed in Fig. 4.
In both of the syntheses routes, the R/O ratio changes with excitation wavelengths. But, comparison of the two syntheses procedures accredits the fact that the phosphor prepared using borohydride method has a better R/O ratio than the solid-state route. The R/O ratio is almost constant in the wavelength region of 220 nm to 240 nm with a value of 0.98 and 0.90 for borohydride and solid-state derived sample, respectively. Another noticeable point is that there is a steep rise in R/O ratio in the shorter wavelength region near VUV range (i.e. 205 and 210 nm). Even in that case, the values are higher (1.72) for the borohydride-derived phosphor, which is favorable for use in PDPs. The higher R/O ratio for borohydride-derived phosphor may be due to the smaller particle size of YBO3:Eu3+. Also, there is no direct correlation between size and color purity.18 In fact, the surface disorder associated with smaller particle size is responsible for the change in R/O values. As a result of smaller particle size, the crystal field environment of europium ions on the surface of particle is of higher complexity than the interior.19 In addition, the europium concentration on the surface and the interior of nano-sized phosphor is different. Higher disordered nature lowers the site symmetry on the surface and this lower site symmetry is not maintained towards the interior of the particle. The contribution of 5D0 → 7F2 transition is more prominent if the site symmetry is lower and thus leads to higher R/O ratio in near VUV range (205 or 210 nm), as VUV radiation penetrates only a part of the nanoparticle surface. However, contribution of both 5D0 → 7F2 and 5D0 → 7F1 transition lead to a decrease the R/O ratio in UV range (220 to 240 nm), as UV radiation penetrates through the particle. So, YBO3:Eu3+ phosphor prepared by our novel method exhibits a better R/O ratio in UV range and considerably higher R/O ratio in near VUV range as compared to solid-state derived phosphor. The observed value of R/O ratio is still higher than the reported value obtained via adopting various solution-based syntheses by modifying different parameters.20,21
The Commission International de I'Eclairage (CIE) 1931 XY chromaticity coordinate graph (XY diagram) of the borohydride synthesized nano-sized YBO3:Eu3+ and conventional solid-state derived bulk phosphor upon different excitation wavelengths are compared in Fig. 5. The CIE 1931 graph contains triangle of R (red), G (green) and B (blue) and the position of a color in the diagram is called chromaticity point of the color. The black and white dot corresponds to solid-state and borohydride derived YBO3:Eu3+ phosphor, respectively. With increase in excitation wavelength, the emission color shifts from pink to reddish orange in both the cases. Such kind of color tuning has never been explored in YBO3:Eu3+ system, although the color tuning in other phosphor materials has been assessed.22–24 In general, the CIE coordinates were calculated by taking into account the visible range (400–700 nm) of the emission spectrum as shown in Fig. 3. The emission spectra consists of a broad peak starting from 250 nm to 450 nm centering at 350 nm and three sharp peaks at 595 nm, 614 nm and 630 nm. The broad nature of peak is mainly due to the contribution of the host i.e. borate group ions, whereas, the three sharp peaks are a result of the contribution of activator i.e. europium ions.18,25,26 The emission mechanism of the phosphor under 205 and 210 nm is indirect emission because of energy transfer from the host to activator.26 So, the contribution of the borate group ions comes to the fore on being excited by high-energy near-VUV radiation and as a result of which a conspicuous emission in 250–450 nm is observed. On the other hand, the activators are directly excited in case of UV radiation (220 nm to 240 nm) and the contribution of borate group is less due to which the intensity of emission in that particular region progressively decreases. This intensity difference manifests itself in the form of a change in the chromaticity coordinate positions in CIE diagram, which shows its position spanning from pink to reddish orange region. However, borohydride derived YBO3:Eu3+ phosphor shows a better chromaticity than solid-state derived phosphor, specifically at lower excitation wavelengths of 205 nm and 210 nm. Although, at higher excitation wavelengths (220, 230 and 240 nm), the chromaticity of borohydride-derived phosphor is comparable with the solid-state derived phosphor. Based on this study, it can be ascertained that tuning of the color intensity of YBO3:Eu3+ phosphors is possible. Efforts for improving the color purity and chromaticity of borohydride derived YBO3:Eu3+ phosphor are under progress.
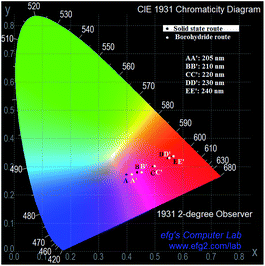 | ||
| Fig. 5 Excitation wavelength variation chromaticity diagram for YBO3:Eu3+ phosphor synthesized by solid-state and borohydride method. | ||
Conclusions
Spherical dumb-bell shaped YBO3:Eu3+ nanophosphor having particle size of 200 nm to 700 nm was successfully prepared by unique borohydride synthesis approach using NaBH4. This gives an affirmative and encouraging indication that the borohydride based novel method of synthesis provides for a higher temperature stability up to 1200 °C. The explicit potential of the borohydride strategy is to prepare YBO3:Eu3+ phosphors with superior characteristics in connection with the color purity described by R/O ratio. The higher R/O ratio (1.72) for near VUV wavelength (205 nm) in borohydride derived phosphor depends on the contribution of 5D0 → 7F2 transition, which is more prominent and may be owing to highly disordered surface with lower site symmetry. However, contribution of both 5D0 → 7F2 and 5D0 → 7F1 transition lead to a decrease in R/O ratio in UV range (220 to 240 nm). Color tuning from pink to reddish orange is possible for YBO3:Eu3+ phosphors by changing the excitation wavelengths from near VUV to UV range due to the contribution of the host and the activator. The findings of the study suggest that this borohydride method is better than the conventional boric acid based solid-state method. Consequently, the borohydride synthesized spherical dumb-bell shaped YBO3:Eu3+ phosphor material could be quite feasible in the display and optics domain.References
- Z. Wei, L. Sun, C. Liao, J. Yin, X. Ziang and C. Yan, J. Phys. Chem. B, 2002, 106, 10610–10617 CrossRef CAS.
- F. Wen, W. Li, Z. Liu, T. Kim, K. Yoo, S. Shin, J. Moon and J. H. Kim, Solid State Commun., 2005, 133, 417–420 CrossRef CAS PubMed.
- H. Ogata, S. Takeshitaa, T. Isobea, T. Sawayama and S. Niikura, Opt. Mater., 2011, 33, 1820–1824 CrossRef CAS PubMed.
- D. Boyer, G. Bertrand and R. Mahiou, J. Lumin., 2003, 104, 229–237 CrossRef CAS.
- A. Nohara, S. Takeshita and T. Isobe, RSC Adv., 2014, 4, 11219–11224 RSC.
- A. Szczeszak, S. Lis and V. Nagirnyi, J. Rare Earths, 2011, 29, 1142–1146 CrossRef CAS.
- R. S. Yadav, R. K. Dutta, M. Kumar and A. C. Pandey, J. Lumin., 2009, 129, 1078–1082 CrossRef CAS PubMed.
- Z. Xu, C. Li, Z. Cheng, C. Zhang, G. Li, C. Peng and J. Lin, CrystEngComm, 2010, 12, 549–557 RSC.
- J. Zhang and J. Lin, J. Cryst. Growth, 2004, 271, 207–215 CrossRef CAS PubMed.
- K. Park and S. W. Nam, Mater. Chem. Phys., 2010, 123, 360–362 CrossRef CAS PubMed.
- D. Zou, Y. Q. Ma, S. B. Qian, G. H. Zheng, Z. X. Dai, G. Li and M. Z. Wu, J. Alloys Compd., 2013, 574, 142–148 CrossRef CAS PubMed.
- X. Jiang, L. Sun, W. Feng and C. Yan, Cryst. Growth Des., 2004, 4, 517–520 CAS.
- L. Chen, H. Cheng, G. Liu and X. Duan, J. Am. Ceram. Soc., 2008, 91, 591–594 CrossRef CAS PubMed.
- P. K. Sharma, R. K. Dutta and A. C. Pandey, J. Appl. Phys., 2012, 112, 054321 CrossRef PubMed.
- U. Rambabu and S.-D. Han, RSC Adv., 2013, 3, 1368–1379 RSC.
- Y. Xu, D. Ma, X. Chen, D. Yang and S. Huang, Langmuir, 2009, 25, 7103–7108 CrossRef CAS PubMed.
- F. Chen, C. Hsu and C. Lu, J. Alloys Compd., 2010, 505, L1–L5 CrossRef CAS PubMed.
- Z. Wei, L. Sun, C. Liao, X. Jiang, C. Yan, Y. Tao, X. Hou and X. Ju, J. Appl. Phys., 2003, 93, 9783–9788 CrossRef CAS PubMed.
- Q. Dong, Y. Wang, Z. Wang, X. Yu and B. Liu, J. Phys. Chem. C, 2010, 114, 9245–9250 CAS.
- X. Zhang, A. Marathe, S. Sohal, M. Holtz, M. Davis, L. J. Hope-Weeks and J. Chaudhuri, J. Mater. Chem., 2012, 22, 6485–6490 RSC.
- X. C. Jiang, C. H. Yan, L. D. Sun, Z. G. Wei and C. S. Liao, J. Solid State Chem., 2003, 175, 245–251 CrossRef CAS.
- N. S. Singh, N. K. Sahu and D. Bahadur, J. Mater. Chem. C, 2014, 2, 548–555 RSC.
- X. Li, S. Zhang, S. Kulinich, Y. Liu and H. Zeng, Sci. Rep., 2014, 4, 4976 Search PubMed.
- P. C. Shen, M. S. Lin and C. F. Lin, Sci. Rep., 2014, 4, 5307 Search PubMed.
- J. H. Kang, W. B. Im, D. C. Lee, J. Y. Kim, D. Y. Jeon, Y. C. Kang and K. Y. Jung, Solid State Commun., 2005, 133, 651–656 CrossRef CAS PubMed.
- J. Dexpert-Ghys, R. Mauricot, B. Caillier, P. Guillot, T. Beaudette, G. Jia, P. A. Tanner and B. M. Cheng, J. Phys. Chem. C, 2010, 114, 6681–6689 CAS.
| This journal is © The Royal Society of Chemistry 2015 |