The impact of 1,2,3-triazoles in the design of functional coatings
Abstract
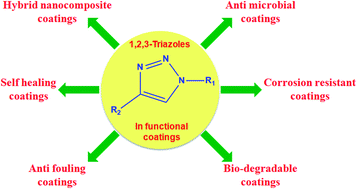
In recent times 1,2,3-triazole rich molecules have gained much importance in the field of polymer and material science because of their excellent properties like strong anti-microbial and anti fouling nature of the triazole ring along with easy synthetic procedures and exceptionally high yield of end product. Generally these molecules are synthesized by azide–alkyne click reaction and this chemistry has potential application in the functionalization of a wide range of inorganic moieties like metal oxide nanoparticles, carbon nanotubes etc. to develop hybrid nanocomposites for high performance materials. Based on the previous reports, this particular review article provides a comprehensive overview of the application of 1,2,3-triazoles in the design of various high performance organic coatings such as anti-corrosive, anti-microbial, self-healing, hybrid nanocomposite, bio degradable etc.

 Please wait while we load your content...
Please wait while we load your content...