Influence of piezoelectric effect on dissolving behavior and stability of ZnO micro/nanowires in solution
Kui Zhanga,
Junjie Qi*a,
Yuan Tiana,
Shengnan Lua,
Qijie Lianga and
Yue Zhang*ab
aSchool of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, People’s Republic of China. E-mail: junjieqi@ustb.edu.cn; yuezhang@ustb.edu.cn
bKey Laboratory of New Energy Materials and Technologies, University of Science and Technology Beijing, Beijing 100083, People’s Republic of China
First published on 25th November 2014
Abstract
We demonstrate for the first time the corrosion behavior of ZnO micro/nanowires under stress. The influence of the piezoelectric effect on the corrosion of ZnO micro/nanowires in acidic and alkaline environments was investigated. The two sides of the bent ZnO micro/nanowires have a significantly different corrosion rate while strain-free ZnO micro/nanowires remain the same. Corrosion behaviors of individually bent ZnO microwires (MWs) have been clearly observed under various strains estimated using the local curvature model. The corrosion phenomena of bent ZnO MWs in acidic and alkaline environments were different. The outer surface of the wire attracts free hydroxide ions and the inner one attracts hydrogen ions from the solution which promotes the chemical reaction due to the effect of the piezoelectric potential which is generated by strain. The experimental results indicated that the corrosion rate is quite sensitive to strain, which provides a recommendation for the design and evaluation of nanodevices that serve in extreme environments.
Introduction
In recent years, ZnO nanomaterials have drawn much attention to the oxide family for numerous applications. A direct band gap of 3.37 eV and large exciton binding energy (60 meV) at room temperature make ZnO a prominent candidate for UV sensors1,2 and light emitting diodes. Due to the unique coupled piezoelectric and semiconducting properties, nanogenerators3 based on nanowires and nanoarrays have been developed. To prevent catastrophic failures people have developed many ways to continuously monitor the state of infrastructure in real time using micro/nanoscale strain sensors4–8 with a high sensitivity and low power requirements or even using self-powered devices.7 Although a lot of progress has been made regarding the mechanical properties of ZnO micro/nanowires,9–12 little work has been conducted on force sensor service conditions such as an acidic or alkaline environment. In recent articles, a lot of biosensors based on ZnO nanowires13–17 were invented owing to their semiconducting properties, high isoelectric point and high specific surface area. Similar to nanogenerators which are used to harvest energy from the environment such as muscle stretching vibrations, a biofluid energy complex with a biosensor can be built into a self-powered wireless nanosensor for implantable biomedical detections. Despite great efforts that have been made on improving the devices’ performance and investigating the biocompatibility, biosafety and even the biodegradability18–20 of ZnO materials, there is no report that addresses the strain effects, which can accelerate the reaction in solution21 when an individual ZnO wire is used as a biosensor or strain sensor in body fluids or other solutions.In this paper, the influence of the piezoelectric effect on the corrosion behavior of individual ZnO MWs has been intensely studied. The strain is induced by bending the ZnO MWs, resulting in a continuous variation of the strain across each single nanowire. The relationship between corrosion rate and strain was obtained. The strain was estimated from the local curvature using a geometrical model. This study reveals a new principle for coupling chemical and mechanical properties which is helpful for the application of biosensors or strain sensors.
Experimental
The ZnO microwires were synthesized, using a common method of chemical vapor deposition, with diameters of 1–10 μm and lengths ranging from several hundred micrometers to several millimeters. Firstly, ZnO powder and carbon powder with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed in a mortar and used as the evaporation source. A p-type silicon (Si) wafer with dimensions of 8 mm × 10 mm was cleaned with acetone, ethanol and ethyl acetate. Then, it was coated with a thin film of Au (10 nm) as the catalyst layer by placing it face-down on a porcelain boat which was loaded with the mixed powders as the source material. The boat was placed in the center of a tube furnace. The whole system was quickly heated to 960 °C under a constant flow of 80 sccm Ar, and then kept at 960 °C for 15 min under a constant flow of 80 sccm Ar mixed with 1 sccm O2. The Si wafer was air-cooled to room temperature and the white product consisting of ZnO MWs was collected from the Si wafer.
1 were mixed in a mortar and used as the evaporation source. A p-type silicon (Si) wafer with dimensions of 8 mm × 10 mm was cleaned with acetone, ethanol and ethyl acetate. Then, it was coated with a thin film of Au (10 nm) as the catalyst layer by placing it face-down on a porcelain boat which was loaded with the mixed powders as the source material. The boat was placed in the center of a tube furnace. The whole system was quickly heated to 960 °C under a constant flow of 80 sccm Ar, and then kept at 960 °C for 15 min under a constant flow of 80 sccm Ar mixed with 1 sccm O2. The Si wafer was air-cooled to room temperature and the white product consisting of ZnO MWs was collected from the Si wafer.
To bend the ZnO MWs under an optical microscope, the ZnO MWs were dispersed onto a cleaned silicon wafer and wires longer than 1 mm with a diameter of about 3 μm were chosen. Each wire was manipulated using a needle tip and the two ends were fixed on the Si substrate with silver paste to keep the bent shape.21–27 Then the silicon wafer was placed in air for 2 hours to let the sliver paste on the silicon wafer dry completely.
To investigate the corrosion behavior of ZnO MWs under strain, the bent wires were carefully put in solution, which was freshly prepared with a concentration of 4 mol L−1 (KOH) or a pH ≈ 5.8–6.2 (HCl). Then, the silicon wafer was removed from the solution and cleaned, and the samples were dried as soon as possible to reduce the influence of DI water. The morphology of the ZnO wires was observed using a Field Emission Scanning Electron Microscope (FE-SEM).
Results and discussion
Fig. 1(a) shows the SEM image of the synthesized sample with a smooth surface. The inset displays a cross-section image of a ZnO wire which appears to have a hexagonal shape. The bent wire fixed on the Si substrate is displayed in Fig. 1(b) and the inset is the high-magnification SEM image of the ZnO wire. The photoluminescence (PL) spectrum of the ZnO wires was measured with a He–Cd laser (325 nm) as the excitation source. In the room-temperature PL spectrum of the nanowires shown in Fig. 1(c), we observed UV emission at 380 nm, corresponding to the near band-edge emission, and a weak green emission at 550 nm, which demonstrated that the prepared ZnO wires have a highly crystalline quality. In order to further characterize the structure of the ZnO wires, a room-temperature Raman spectrum of the ZnO wires was taken which is illustrated in Fig. 1(d). The peaks at 98, 331, 438 and 582 cm−1 indicated that the ZnO MWs had a wurtzite hexagonal ZnO structure. Fig. 1(e) depicts the XRD pattern of the nanowires. The diffraction peaks can be readily indexed to a hexagonal structure. The HRTEM and low-magnification TEM images of a ZnO wire and the corresponding select-area electron diffraction (SAED) pattern are shown in Fig. 1(f), which indicate that each micro/nanowire is a wurtzite-structured single crystal growing along the [0001] direction.A strain-free ZnO wire with a diameter of about 650 nm was prepared on a Si wafer, as shown in Fig. 2(a). After interacting with 4 M KOH solution for 5, 15 or 25 minutes, the morphology of the ZnO wire was examined, as exhibited in Fig. 2(b)–(d), respectively. The wire became coarse and the shape was not regular hexagonal anymore, which could be aggravated by increasing the reaction time. The chemical reaction in this case can be expressed using the equation:
| ZnO(s) + 2OH− → ZnO22− + H2O, | (1) |
| ZnO(s) + 2H+ → Zn2+ + H2O. | (2) |
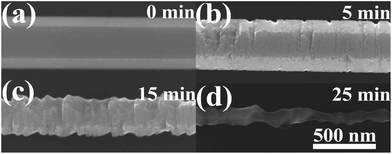 | ||
| Fig. 2 SEM images of an individual ZnO wire which interacted with 4 M KOH solution for different lengths of time: (a) 0, (b) 5, (c) 15 and (d) 25 minutes. The scale bar in (d) is the same for (a–c). | ||
 | ||
| Fig. 3 SEM images of a ZnO wire which interacted with HCl solution of pH ≈ 5.8–6.2 for different lengths of time: (a) 0, (b) 5, (c) 15 and (d) 25 minutes. The scale bar in (d) is the same for (a–c). | ||
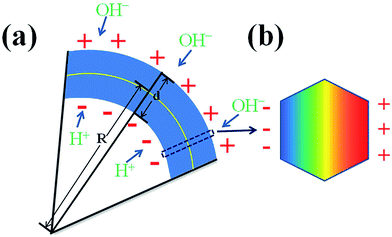
For an ideal curved nanowire, there are enough reasons to deem that the outer (inner) edge of the nanowire endures a tensile (compressive) strain, while the center of the nanowire is free of any strain, as depicted in Fig. 4(a) and (b).
In order to obtain the strain rate of the different areas, the geometric model shown in Fig. 4(b) was used. The strain rate (ε) can be calculated using the local curvature radius (ρ) and the diameter d of the wire.26
 | (3) |
In this paper, two different bent MWs were prepared. We put the bent wires of Fig. 5(A) and 6(A) in a two-dimensional coordinate system and ten points on each wire have been taken to determine the curves’ equations on the basis of the shapes, as shown in Fig. 4(c) and (d):
| y = 79.53 − 0.40x + 0.0012x2 | (4) |
| y = 79.36 − 0.58x + 0.0019x2 | (5) |
According to the above equation, we can measure the local curvature radius (ρ) of the wire with the formula:
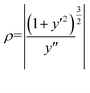 | (6) |
where y′ is the first and y′′ is the second derivative. The insets of Fig. 4(c) and (d) show the corresponding ρ values of the rectangular areas in Fig. 5(A) and 6(A).
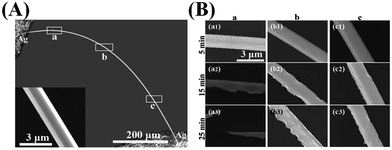
In order to observe the influence of the strain effect on the corrosion behavior of the ZnO wire, a bent wire with a diameter of about 3.8 μm and a length of about 1 mm was prepared, as shown in Fig. 5(A) where the inset is the corresponding high-magnification SEM image. The strain rates of the ZnO wire in the different rectangular areas ‘a–d’ are 0.96, 0.37, 0.06 and 0.03%, respectively. Fig. 5(B) displays the high-magnification SEM images of the different regions in (A) interacting with KOH solution for 5, 15, 25 or 35 minutes. When the etching time reaches 35 min the rectangular area of ‘a’ (Fig. 5(A)) cracked. The diameter of the ZnO wire decreased faster in KOH solution when the wire suffered a larger strain, and the outer surface of the wire corrodes more strongly and more irregularly than the inner side of the wire, which becomes even more serious when the strain increases.
To confirm the phenomenon, we designed another experiment. Fig. 6(A) shows a low-magnification SEM image of a bent ZnO wire which is about 0.8 mm long with a diameter of about 1.65 μm, and the high-magnification SEM image of one of the rectangular areas in the inset of Fig. 6(A), which was corroded in HCl solution for different lengths of time, as shown in Fig. 6(B). When the etching time reached 25 min the rectangular area of ‘a’ (Fig. 6(A)) cracked. The corrosion behavior is similar to that of the wire in KOH solution except that the inner surface of the wire corrodes more strongly and more irregularly than the outer side.
The diameter reduction rates of the two bent wires with different strain rates and reaction times are presented in Fig. 7(a) and (b). The diameter of ZnO wire decreased with increasing etching time, and the diameter change was more pronounced when the wire was under a higher strain. The change in diameter of the ZnO wires follows the same law in the two solutions.
 | ||
| Fig. 7 Diameter reduction rates of the two bent wires corresponding to the different regions in Fig. 5 and 6 in different solutions: (a) KOH, and (b) HCl. | ||
The ZnO wire produces a piezoelectric effect when it suffers strain,3,28,29 positive (negative) charges were generated on the outer (inner) side of the wire surface which vanished only when the strain was released, as depicted in Fig. 8(a). The piezoelectric effect accelerates the corrosion rate of ZnO wires in solution. The outer surface of the wire attracts free hydroxide ions and the inner one attracts hydrogen ions in solution which can promote the chemical reaction, shown in eqn (1) and (2). In addition, the lattice constant along the c-axis of the bent wire changed; the outer surface of the wire became larger while the inner surface became smaller, which accelerated the chemical reaction.
Conclusions
In conclusion, we studied the piezoelectric effect on the corrosion behavior of ZnO wires in acidic and alkaline environments. We chose KOH and HCl solutions as the simulated environments. A strain-free ZnO wire corroded almost symmetrically in solution, while the bent wire corroded quite differently and the failure phenomenon appears faster under larger strain due to a higher piezoelectric potential. These findings may remind future investigators to consider this phenomenon when using ZnO-based devices as biosensors, strain sensors, etc. To the authors’ knowledge, this is the first report on the observation of stress corrosion in ZnO nanowires and will hopefully inspire the research of stress corrosion in piezoelectric materials.Acknowledgements
This work was supported by the National Major Research Program of China (2013CB932600), the Program of International S&T Cooperation (2012DFA50990), NSFC (51232001, 51172022), the Research Fund of Co-construction Program from Beijing Municipal Commission of Education, the Fundamental Research Funds for the Central Universities, and the Program for Changjiang Scholars and Innovative Research Team in University.Notes and references
- D. Gedamu, I. Paulowicz, S. Kaps, O. Lupan, S. Wille, G. Haidarschin, Y. K. Mishra and R. Adelung, Adv. Mater., 2014, 26, 1541–1550 CrossRef CAS PubMed.
- J. Qi, X. Hu, Z. Wang, X. Li, W. Liu and Y. Zhang, Nanoscale, 2014, 6(11), 6025–6029 RSC.
- Y. Gao and Z. L. Wang, Nano Lett., 2007, 7, 2499–2505 CrossRef CAS PubMed.
- Q. Liao, M. Mohr, X. Zhang, Z. Zhang, Y. Zhang and H. J. Fecht, Nanoscale, 2013, 5, 12350–12355 RSC.
- S. Lu, J. Qi, Z. Wang, P. Lin, S. Liu and Y. Zhang, RSC Adv., 2013, 3, 19375 RSC.
- I. Shakir, Z. Ali, J. Bae, J. Park and D. J. Kang, RSC Adv., 2014, 4, 6324 RSC.
- Z. Wang, J. Qi, X. Yan, Q. Zhang, Q. Wang, S. Lu, P. Lin, Q. Liao, Z. Zhang and Y. Zhang, RSC Adv., 2013, 3, 17011 RSC.
- H. Gullapalli, V. S. Vemuru, A. Kumar, A. Botello-Mendez, R. Vajtai, M. Terrones, S. Nagarajaiah and P. M. Ajayan, Small, 2010, 6, 1641–1646 CrossRef CAS PubMed.
- P. Li, Q. Liao, S. Yang, X. Bai, Y. Huang, X. Yan, Z. Zhang, S. Liu, P. Lin, Z. Kang and Y. Zhang, Nano Lett., 2014, 14, 480–485 CrossRef CAS PubMed.
- R. Agrawal, B. Peng and H. D. Espinosa, Nano Lett., 2009, 9, 4177–4183 CrossRef CAS PubMed.
- F. Xu, Q. Qin, A. Mishra, Y. Gu and Y. Zhu, Nano Res., 2010, 3, 271–280 CrossRef CAS PubMed.
- A. Asthana, K. Momeni, A. Prasad, Y. K. Yap and R. S. Yassar, Nanotechnology, 2011, 22, 265712 CrossRef CAS PubMed.
- R. Yu, C. Pan, J. Chen, G. Zhu and Z. L. Wang, Adv. Funct. Mater., 2013, 23, 5868–5874 CrossRef CAS.
- F. Patolsky, G. Zheng and C. M. Lieber, Anal. Chem., 2006, 78, 4260–4269 CrossRef CAS.
- J. M. Moon, Y. Hui Kim and Y. Cho, Biosens. Bioelectron., 2014, 57, 157–161 CrossRef CAS PubMed.
- R. Yakimova, L. Selegård, V. Khranovskyy, R. Pearce, A. Lloyd Spetz and K. Uvdal, Front. Biosci., Elite Ed., 2012, 4, 254–278 CrossRef PubMed.
- Y. Zhao, X. Yan, Z. Kang, P. Lin, X. Fang, Y. Lei, S. Ma and Y. Zhang, Microchim. Acta, 2013, 180, 759–766 CrossRef CAS.
- J. Zhou, N. S. Xu and Z. L. Wang, Adv. Mater., 2006, 18, 2432–2435 CrossRef CAS.
- W. Zhou, X. Dai, T. M. Fu, C. Xie, J. Liu and C. M. Lieber, Nano Lett., 2014, 14, 1614–1619 CrossRef CAS PubMed.
- Z. Li, R. Yang, M. Yu, F. Bai, C. Li and Z. L. Wang, J. Phys. Chem. C, 2008, 112, 20114–20117 CAS.
- S. Xu, W. Guo, S. Du, M. M. Loy and N. Wang, Nano Lett., 2012, 12, 5802–5807 CrossRef CAS PubMed.
- X. Han, L. Kou, Z. Zhang, Z. Zhang, X. Zhu, J. Xu, Z. Liao, W. Guo and D. Yu, Adv. Mater., 2012, 24, 4707–4711 CrossRef CAS PubMed.
- X. Han, L. Kou, X. Lang, J. Xia, N. Wang, R. Qin, J. Lu, J. Xu, Z. Liao, X. Zhang, X. Shan, X. Song, J. Gao, W. Guo and D. Yu, Adv. Mater., 2009, 21, 4937–4941 CrossRef CAS PubMed.
- C. P. Dietrich, M. Lange, F. J. Klüpfel, H. von Wenckstern, R. Schmidt-Grund and M. Grundmann, Appl. Phys. Lett., 2011, 98, 031105 CrossRef PubMed.
- J. Chen, G. Conache, M. E. Pistol, S. M. Gray, M. T. Borgstrom, H. Xu, H. Q. Xu, L. Samuelson and U. Hakanson, Nano Lett., 2010, 10, 1280–1286 CrossRef CAS PubMed.
- H. Xue, N. Pan, M. Li, Y. Wu, X. Wang and J. G. Hou, Nanotechnology, 2010, 21, 215701 CrossRef PubMed.
- Z. M. Liao, H. C. Wu, Q. Fu, X. Fu, X. Zhu, J. Xu, I. V. Shvets, Z. Zhang, W. Guo, Y. Leprince-Wang, Q. Zhao, X. Wu and D. P. Yu, Sci. Rep., 2012, 2, 452 Search PubMed.
- Z. L. Wang, Adv. Mater., 2007, 19, 889–892 CrossRef CAS.
- S. Yang, L. Wang, X. Tian, Z. Xu, W. Wang, X. Bai and E. Wang, Adv. Mater., 2012, 24, 4676–4682 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |