A self-powered ultraviolet photodetector based on solution-processed p-NiO/n-ZnO nanorod array heterojunction
Abstract
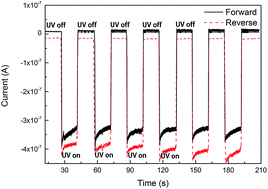
We report fabrication of an all inorganic, self-powered and rapid-response ultraviolet (UV) photodetector using solution-processed p-NiO/ZnO-nanorod array heterojunction. The device exhibited a fast binary-response with a rise time of 0.23 s and decay time of 0.21 s. A large responsivity of 0.44 mA W−1 was achieved for a 0.4 mW cm−2 UV light irradiation at a zero-bias voltage. The self-powered performance could be attributed to the proper built-in electric field between ZnO and NiO arising from the well-aligned energy-band structure of the device, which gives rise to a photovoltaic effect.

 Please wait while we load your content...
Please wait while we load your content...